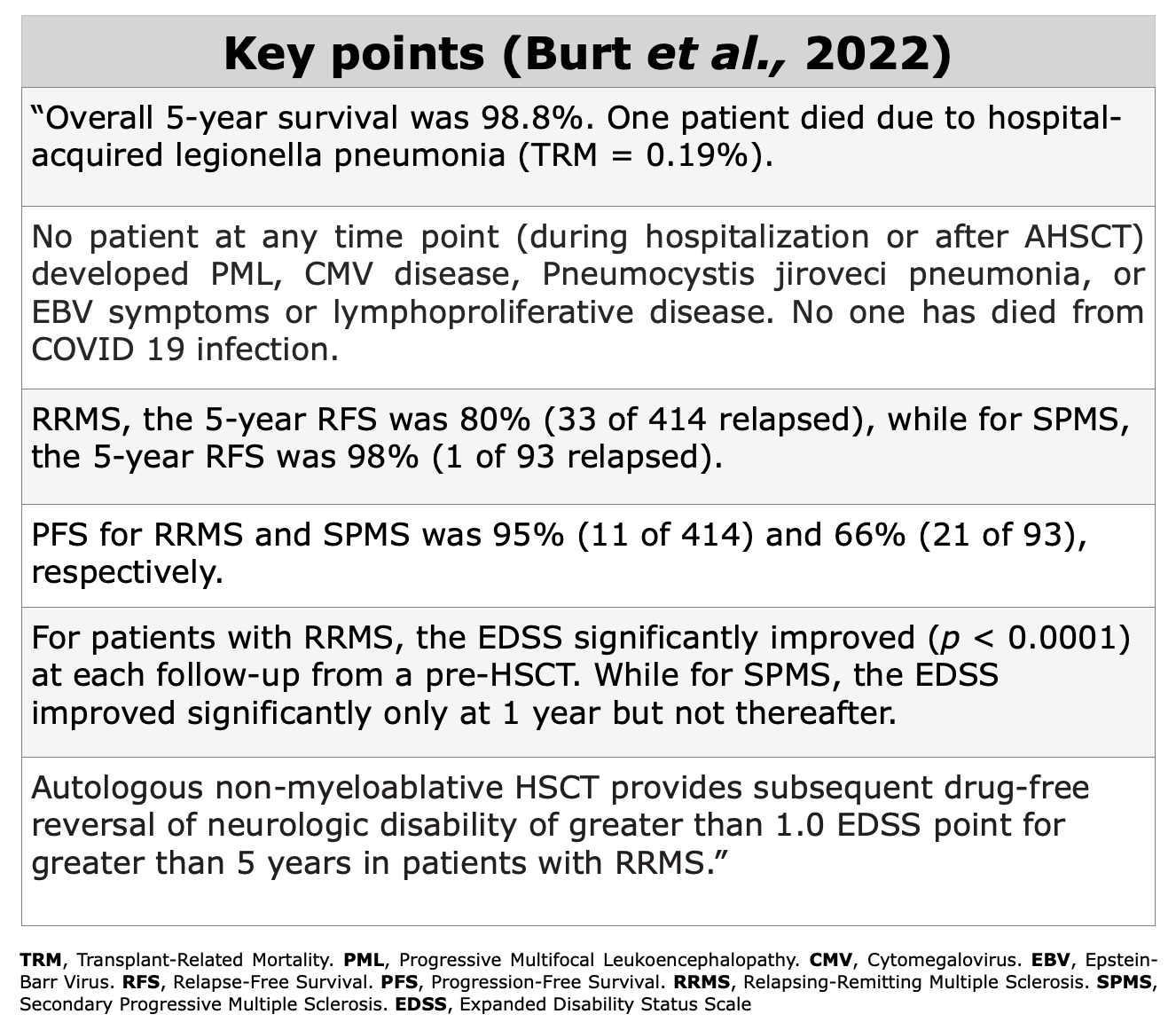
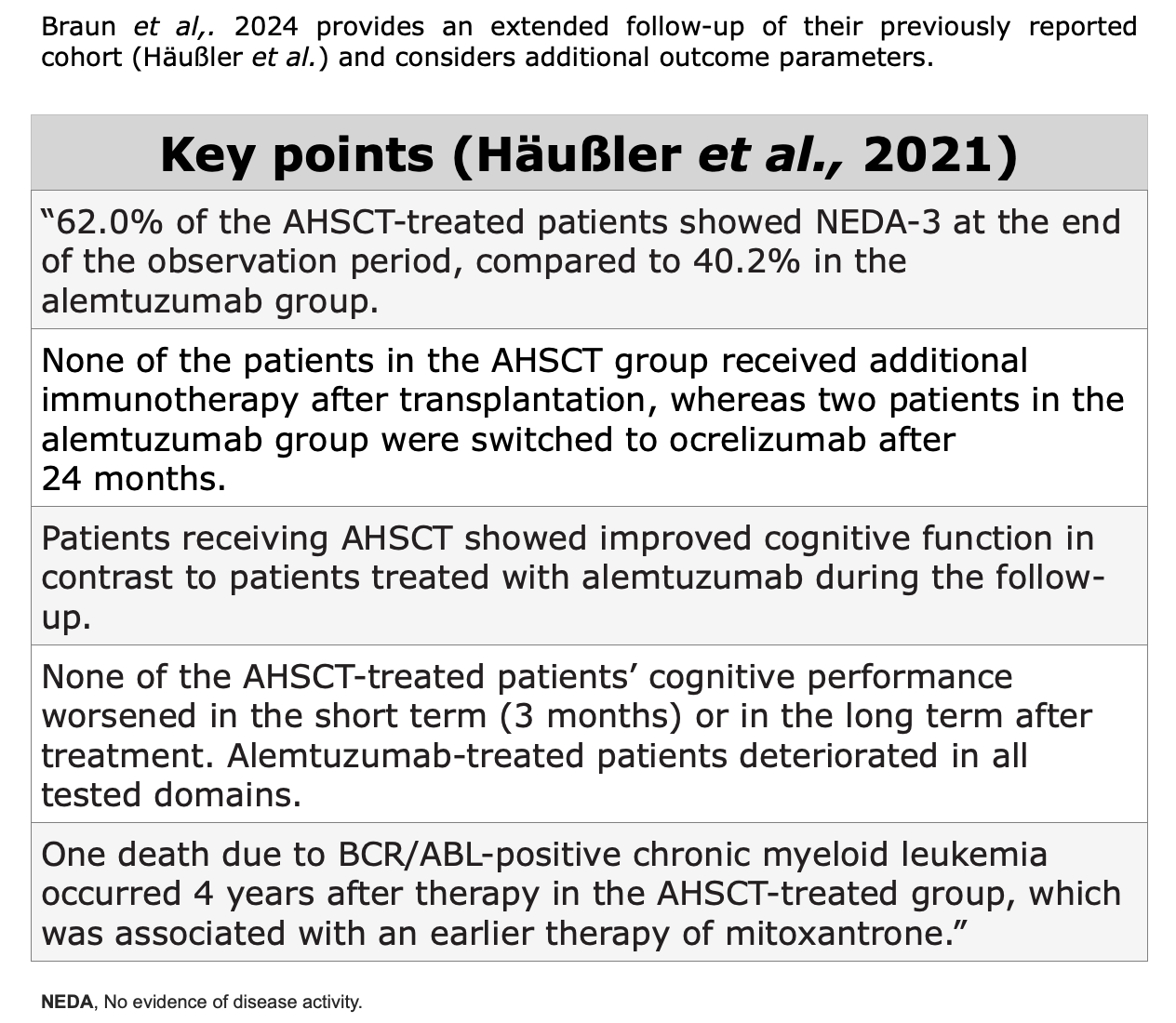
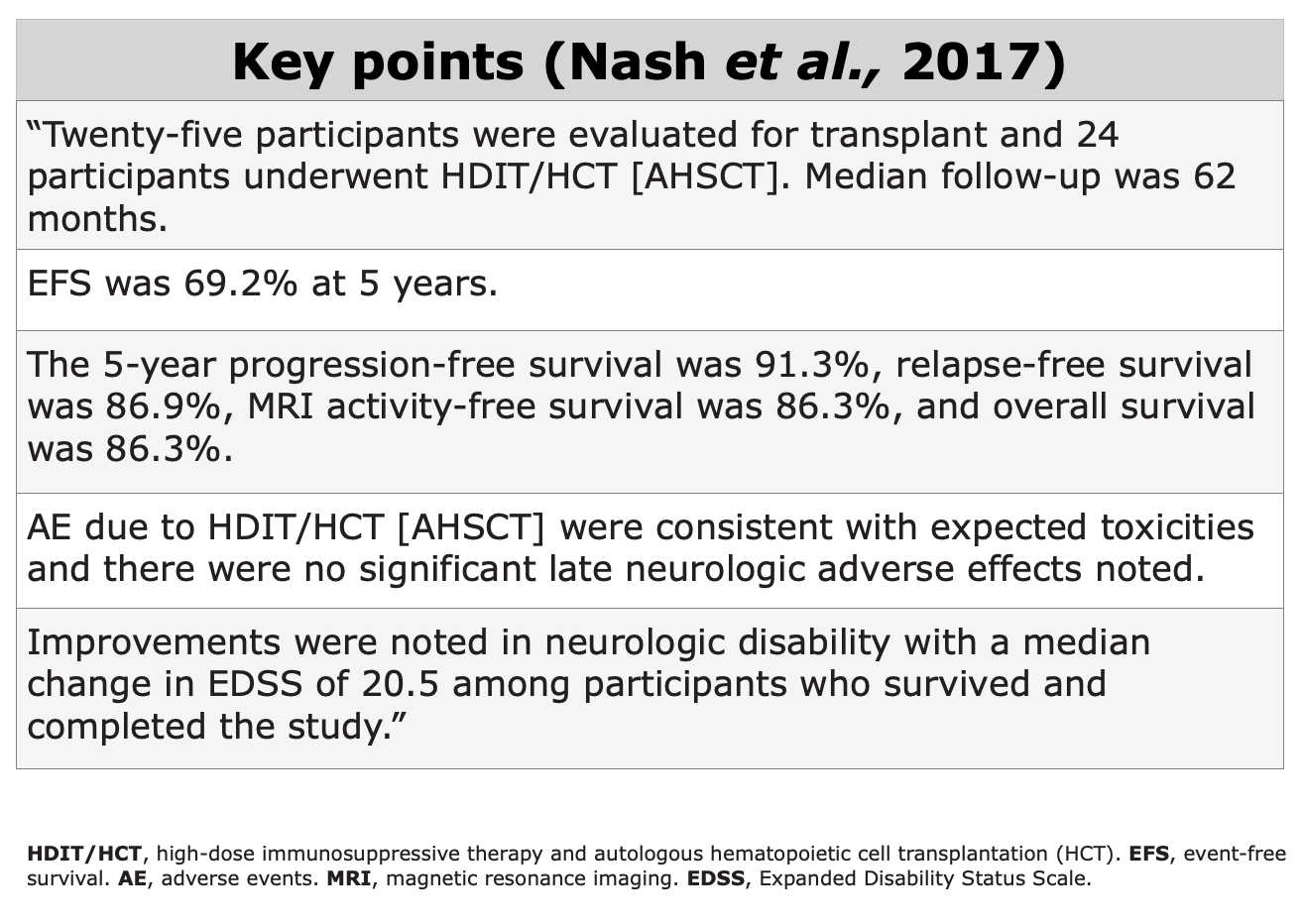
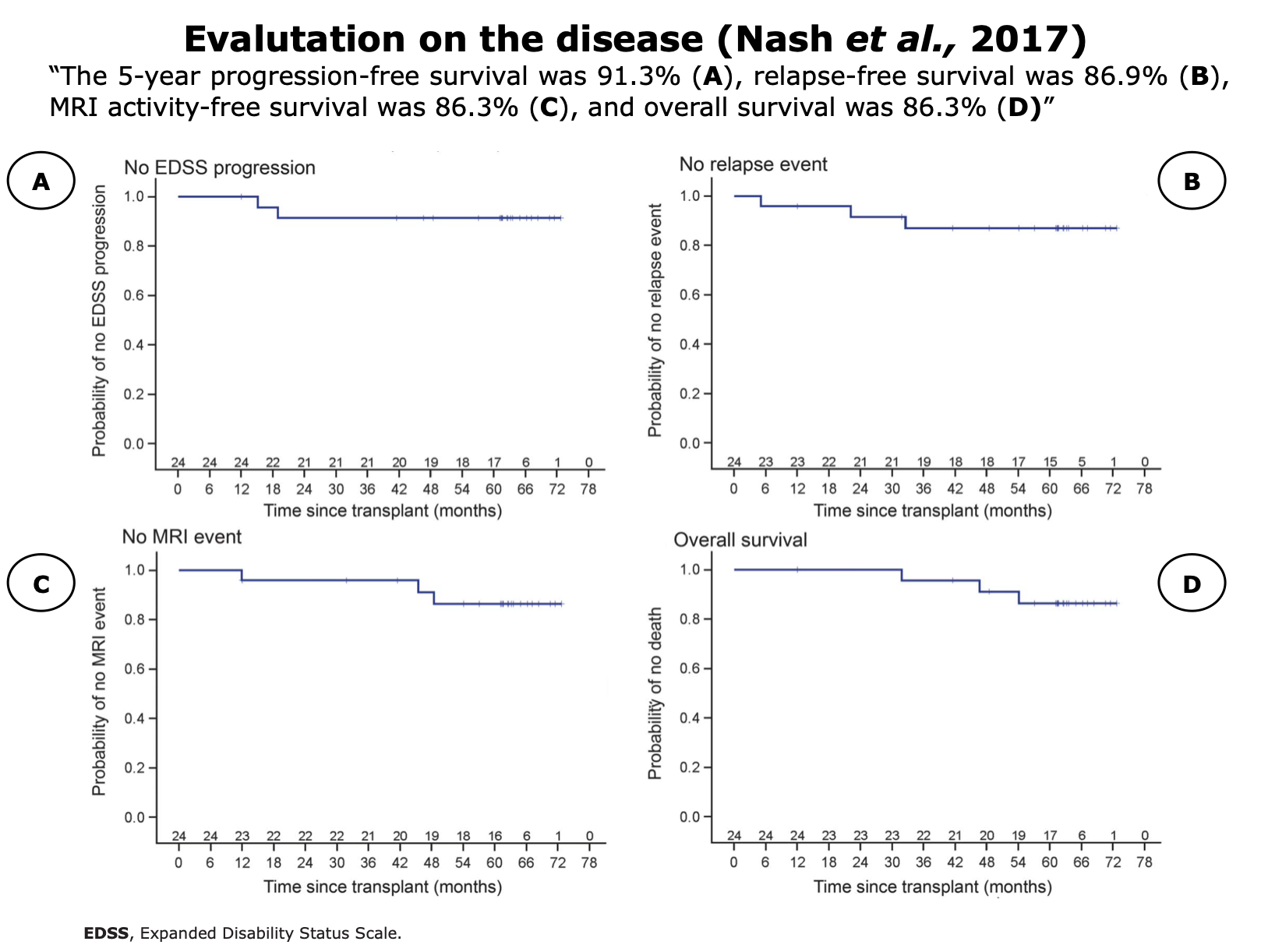
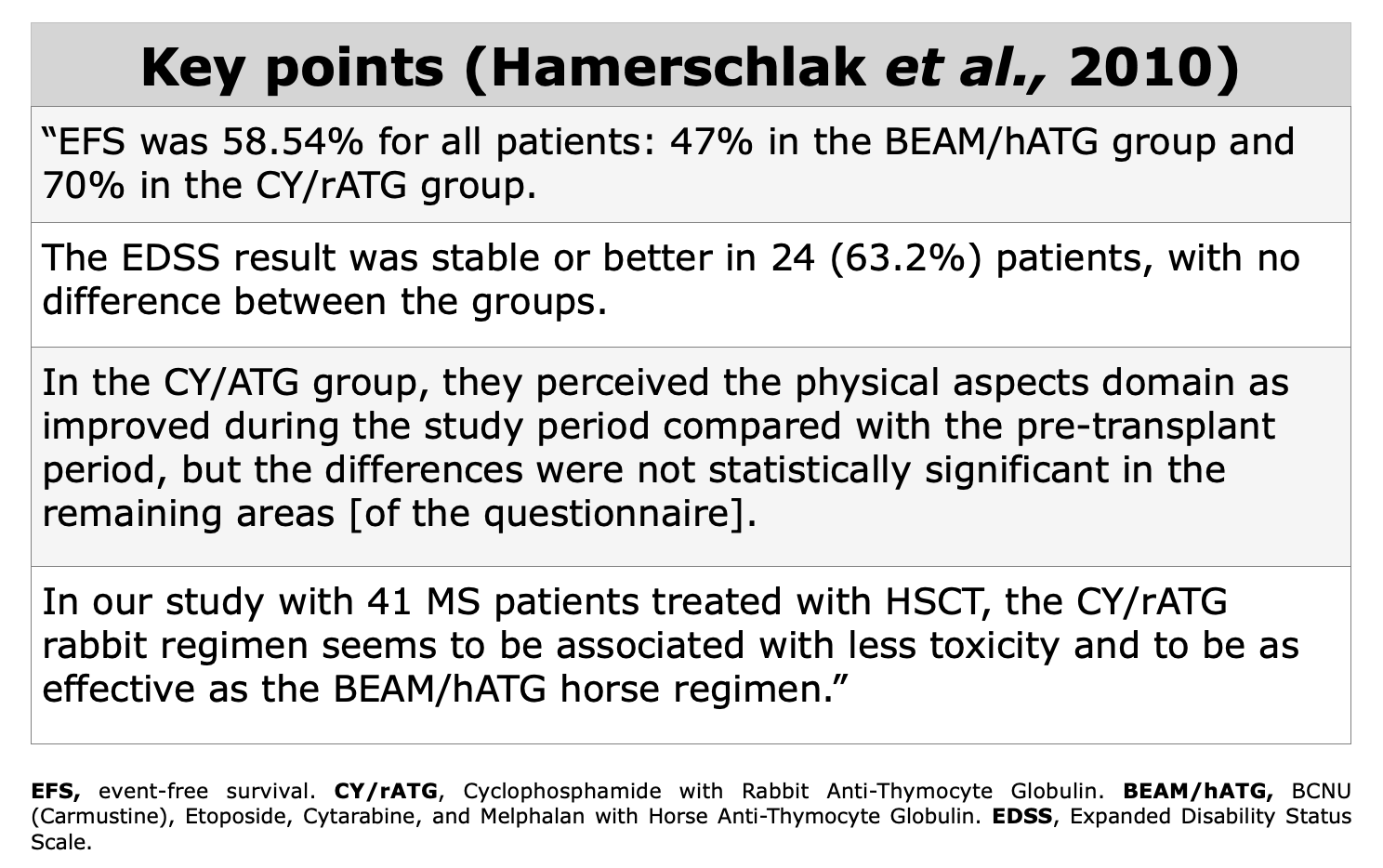
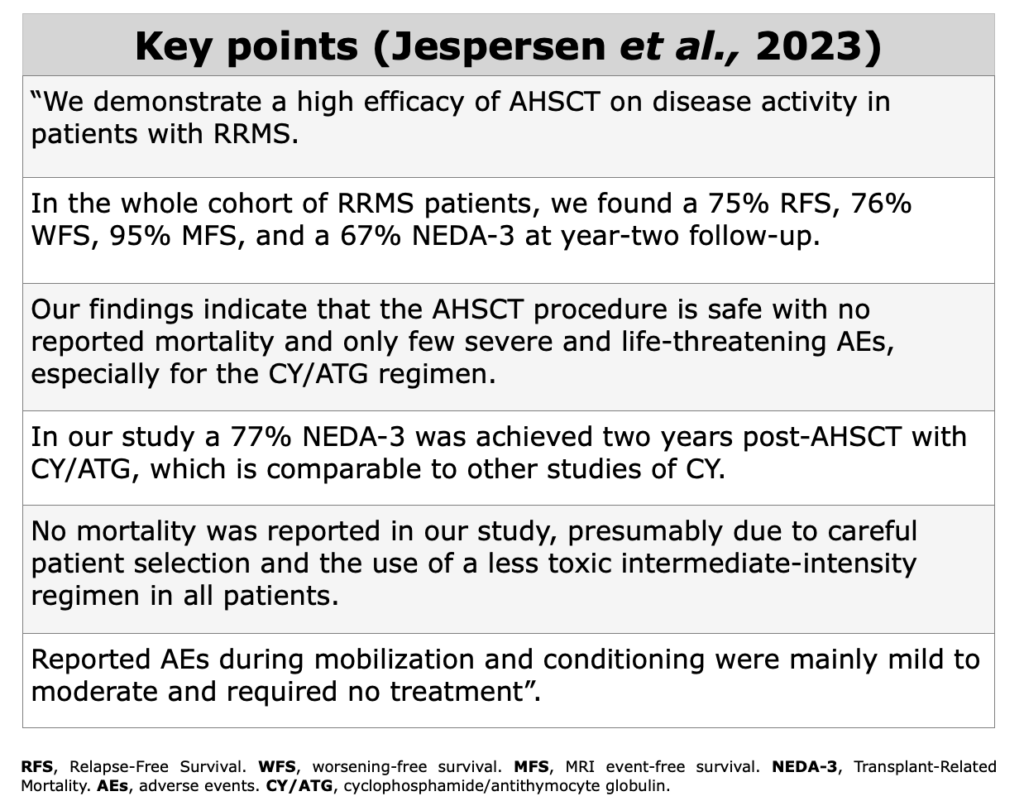
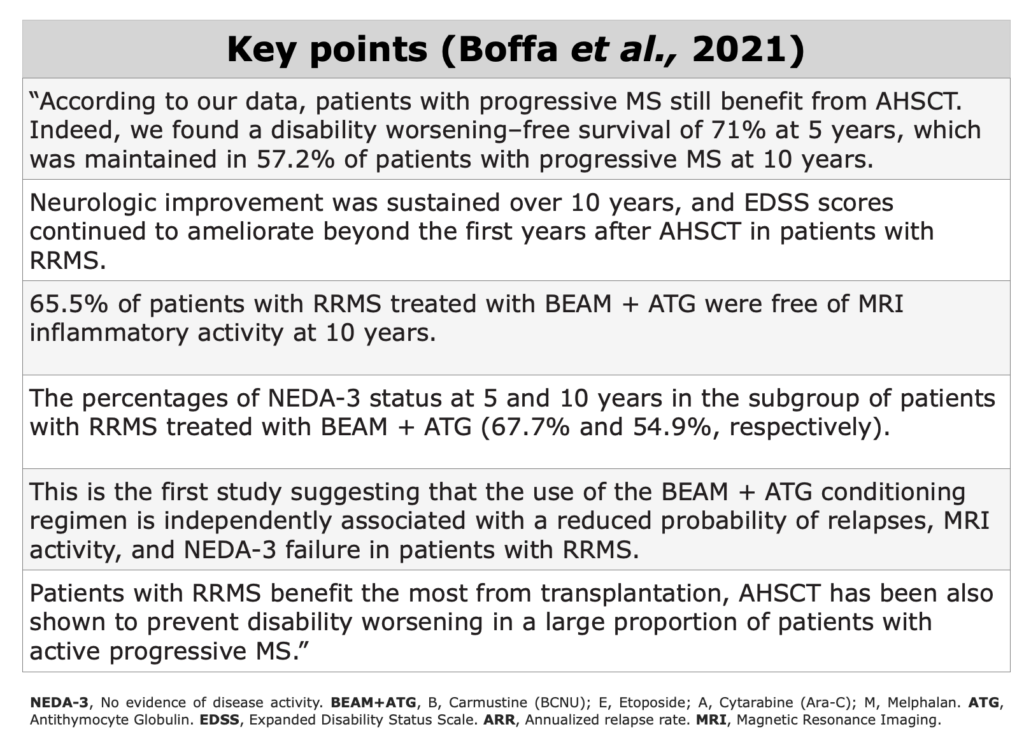
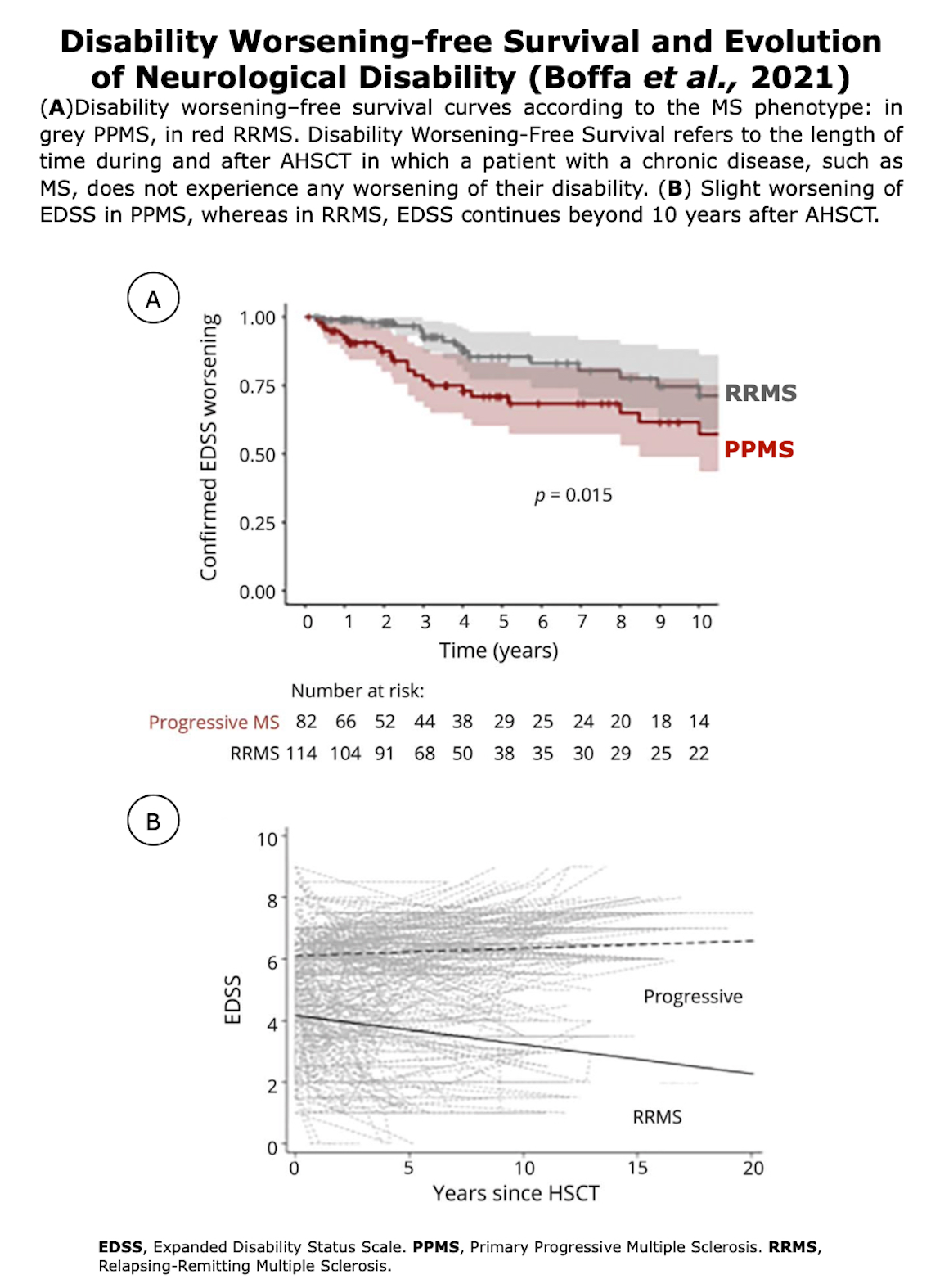
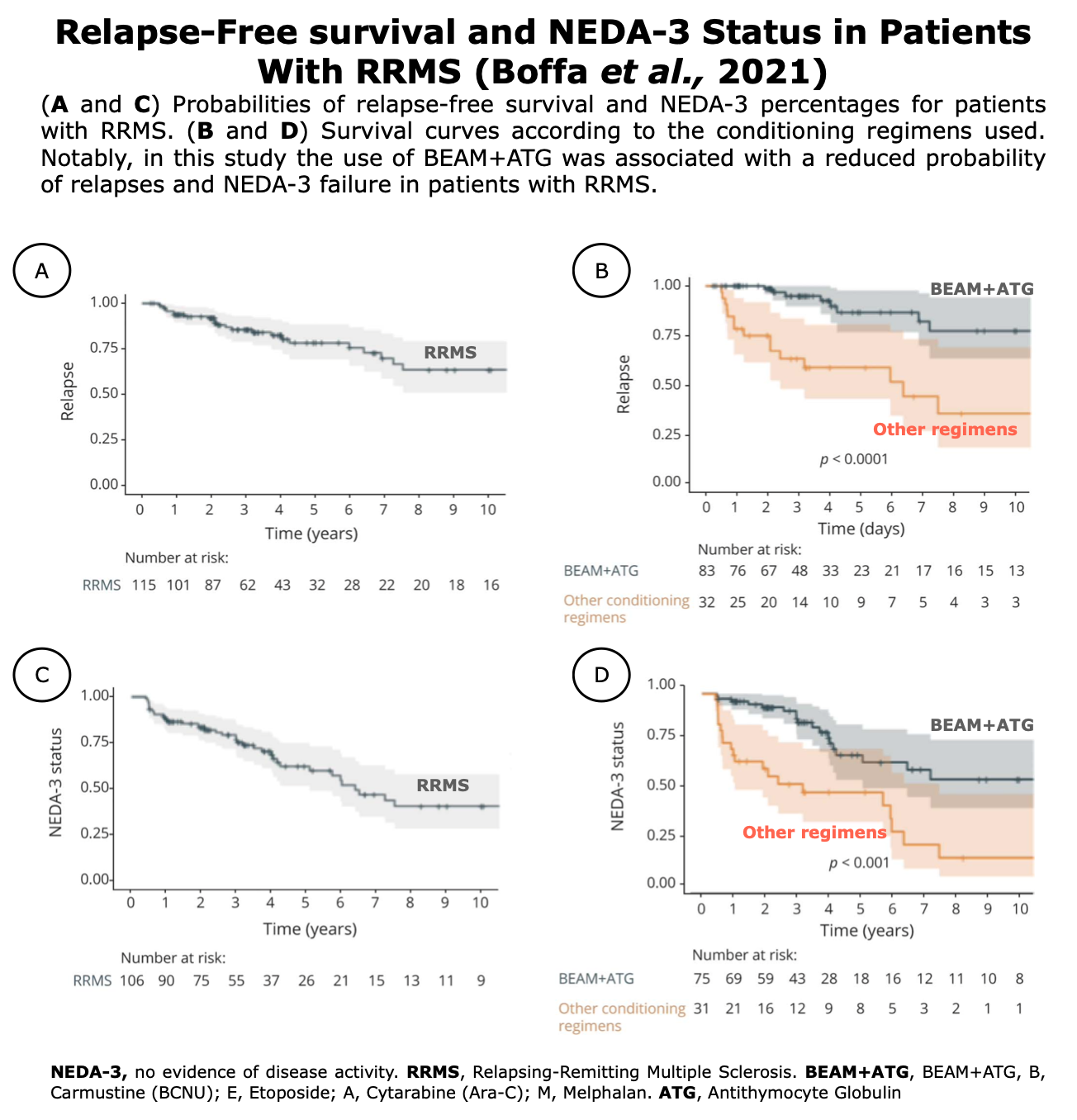
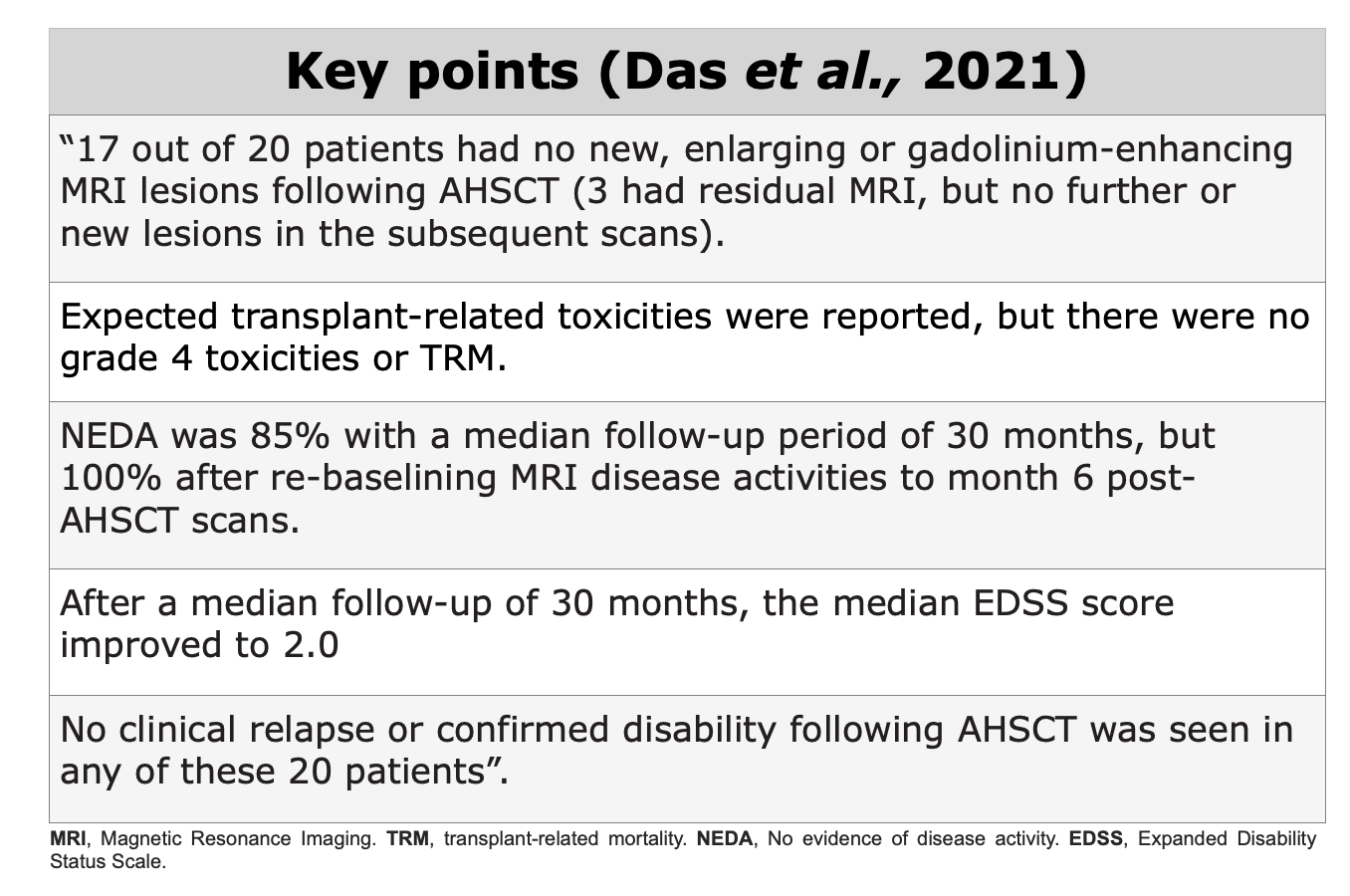
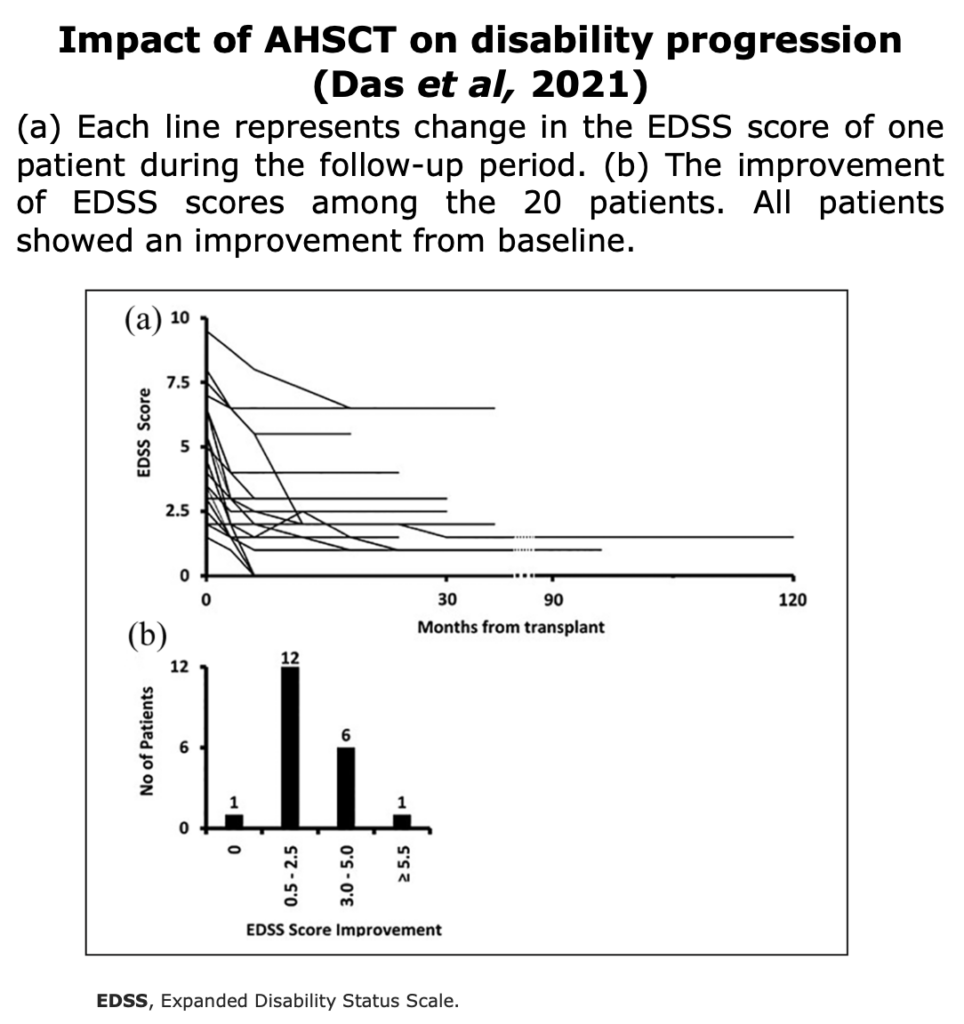
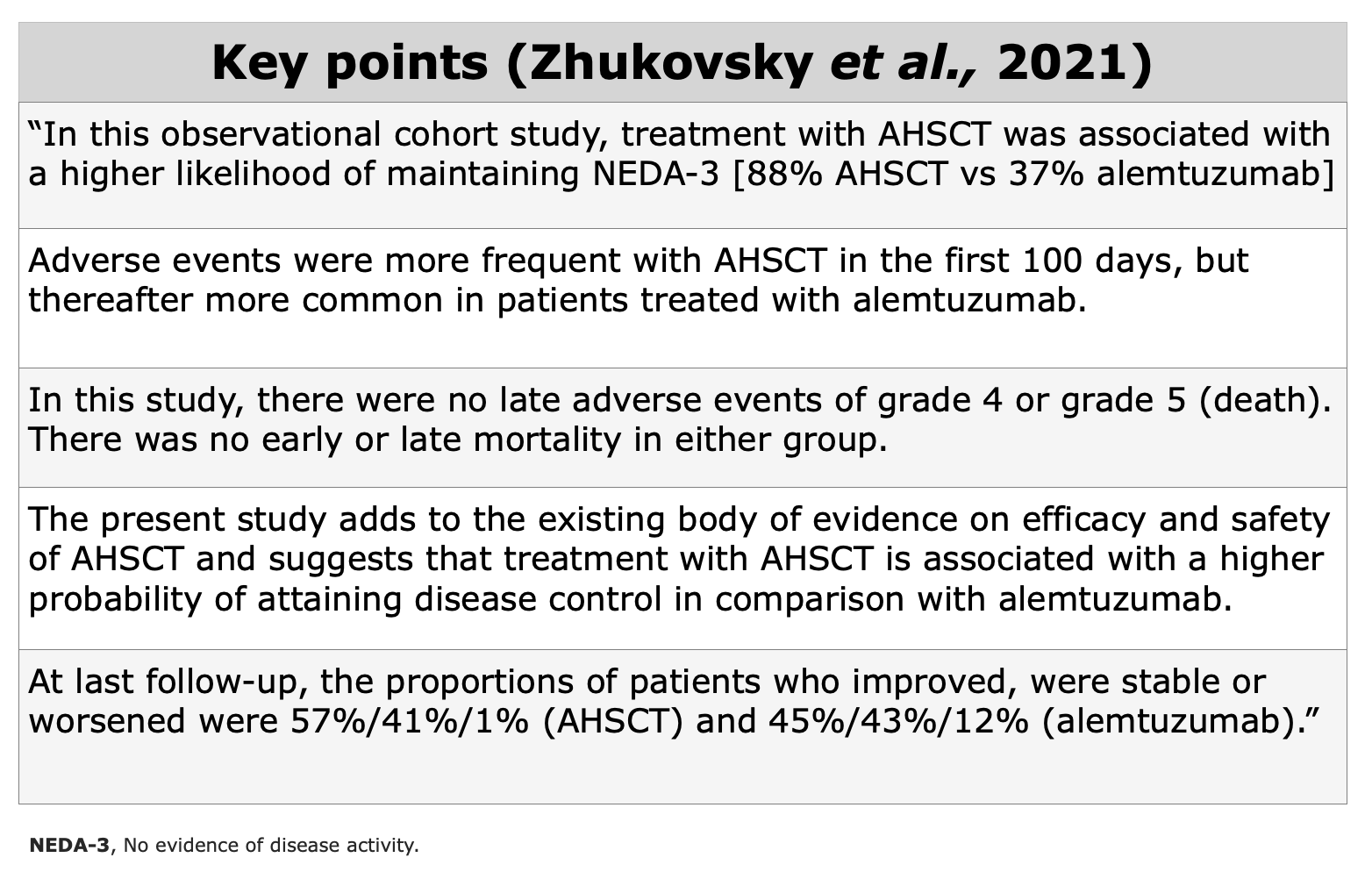
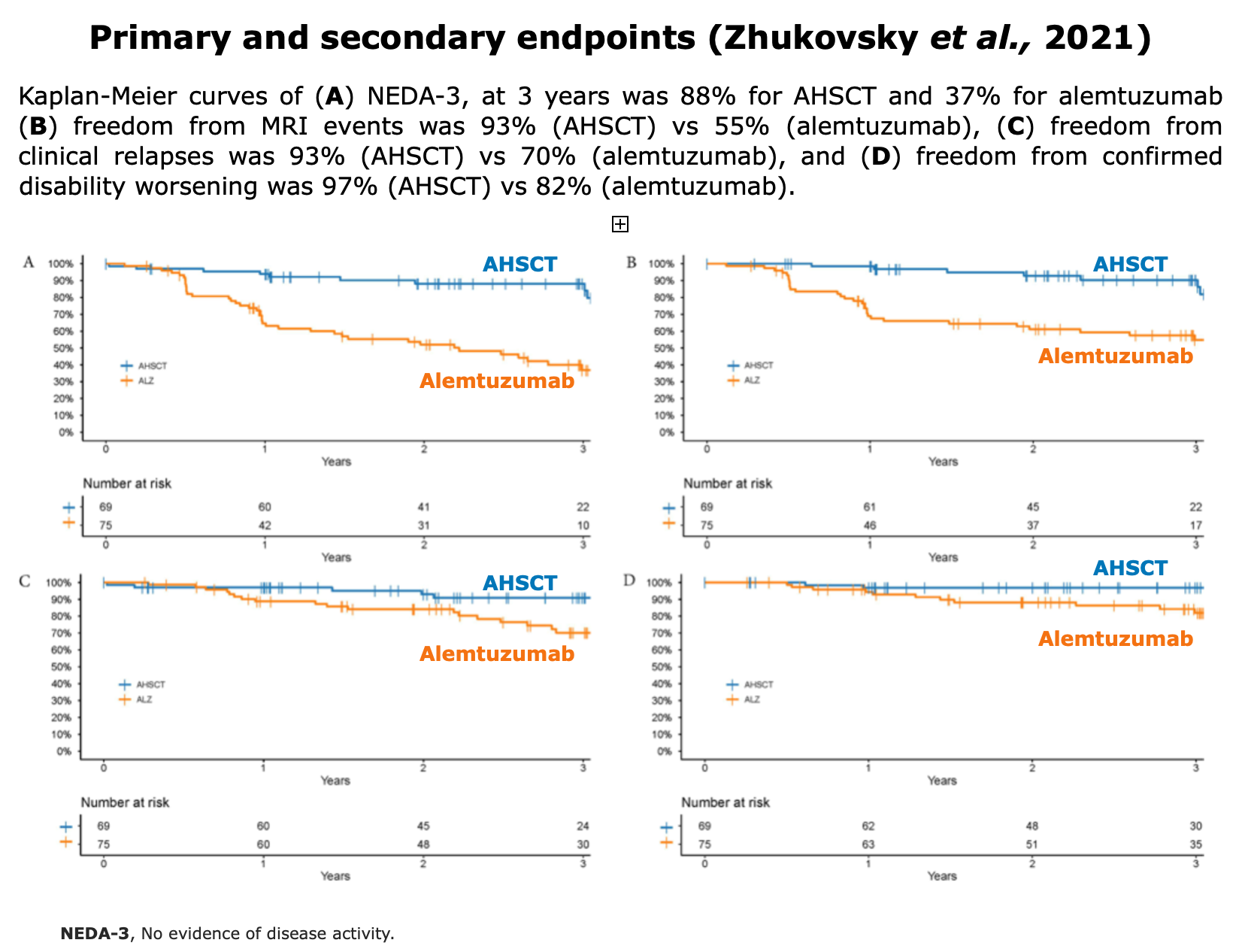
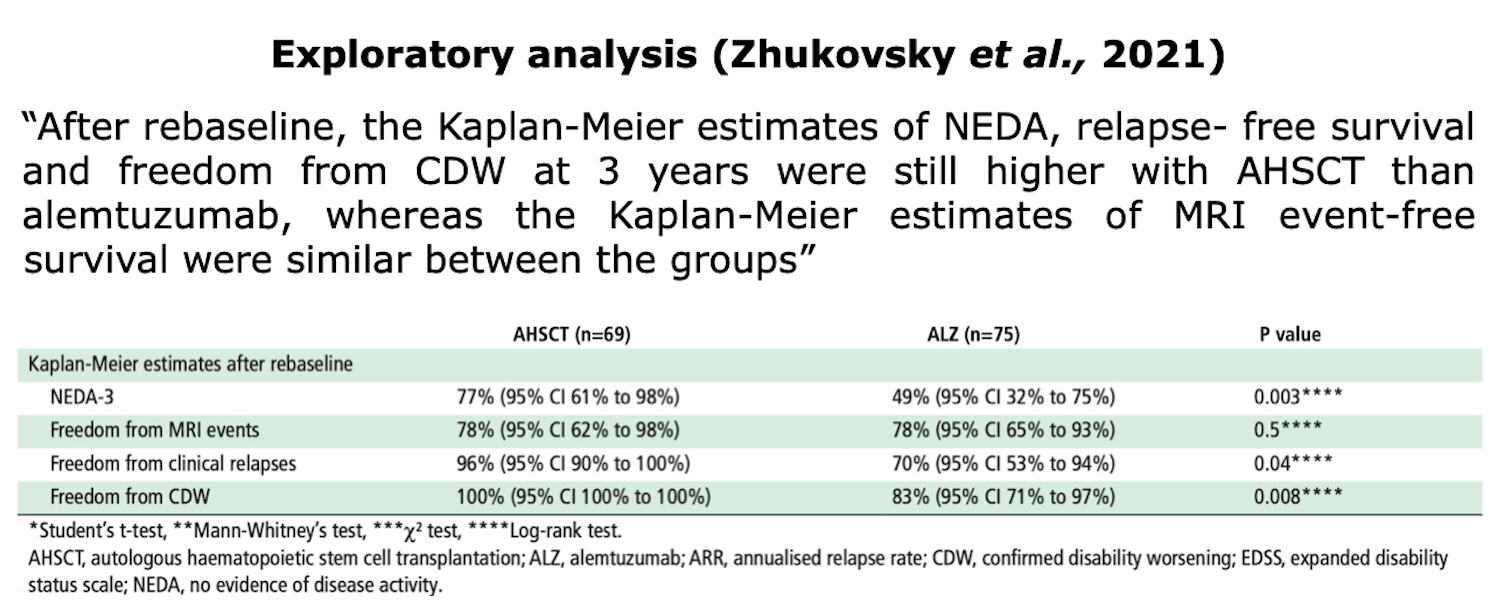
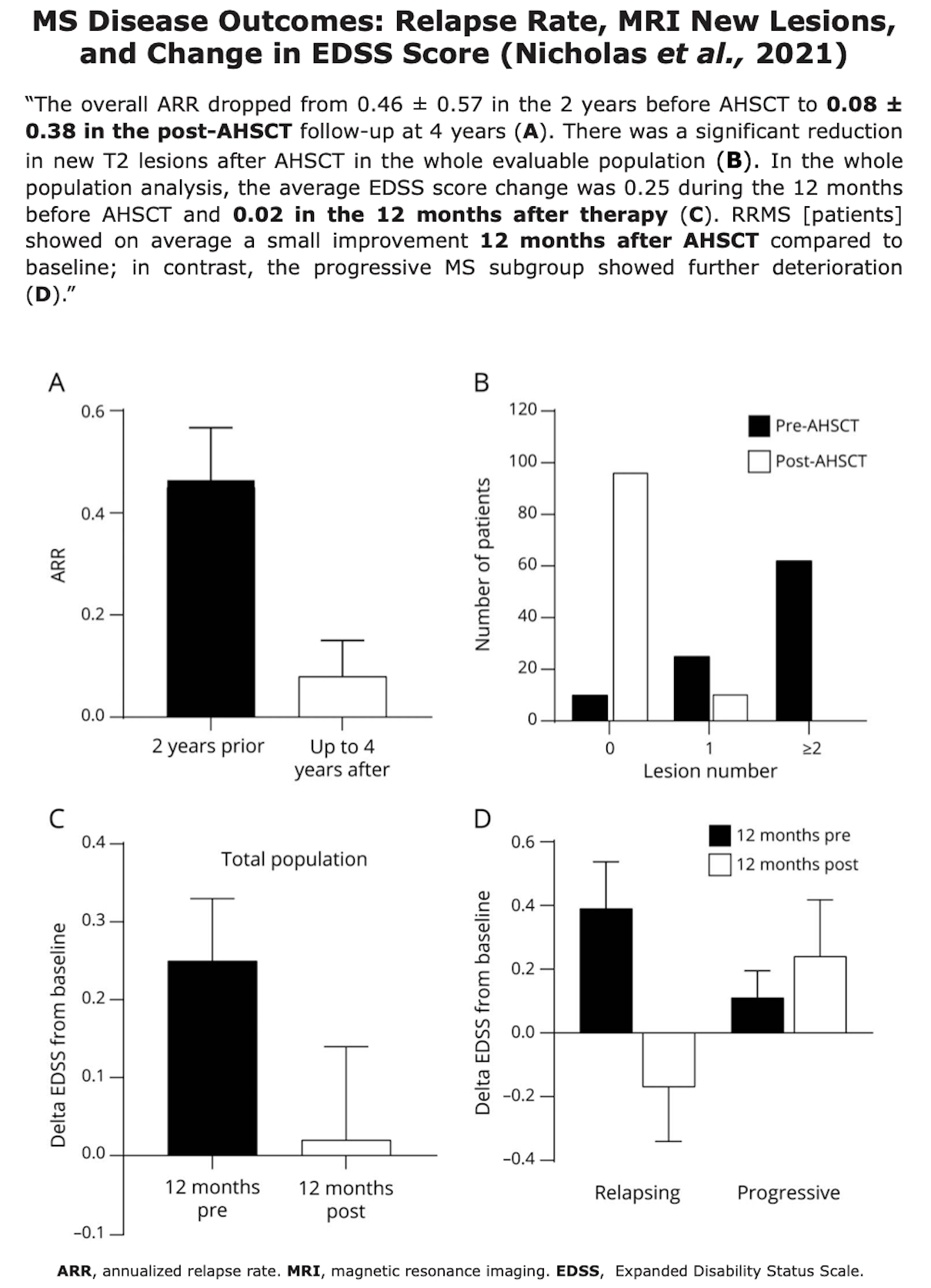
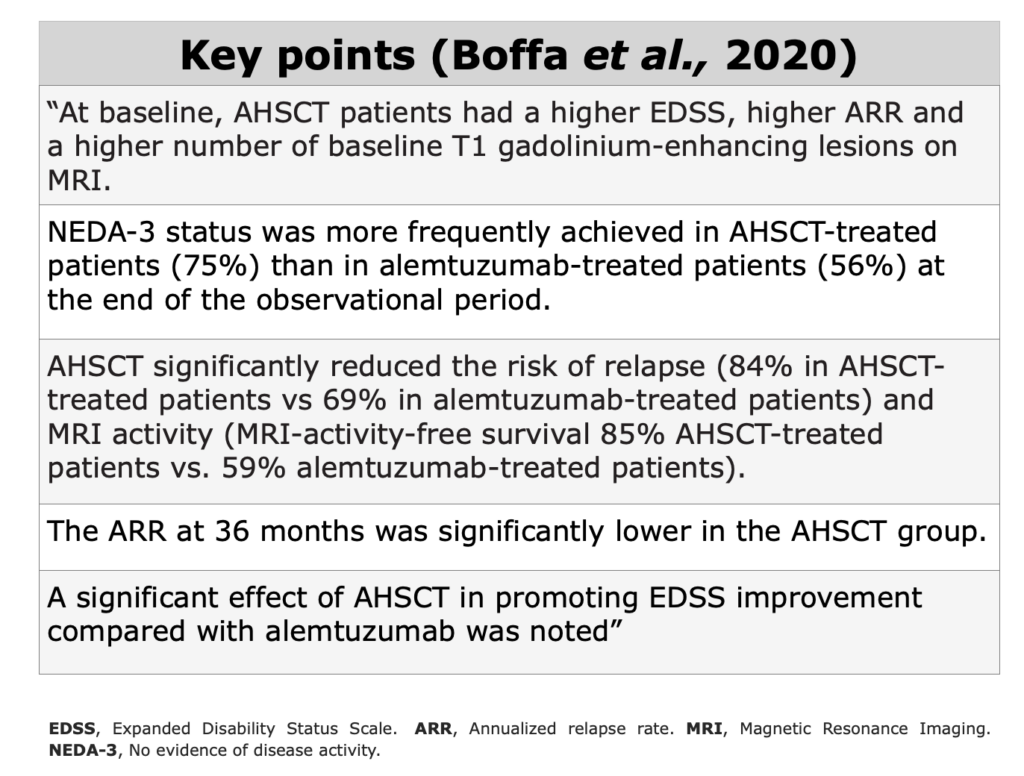
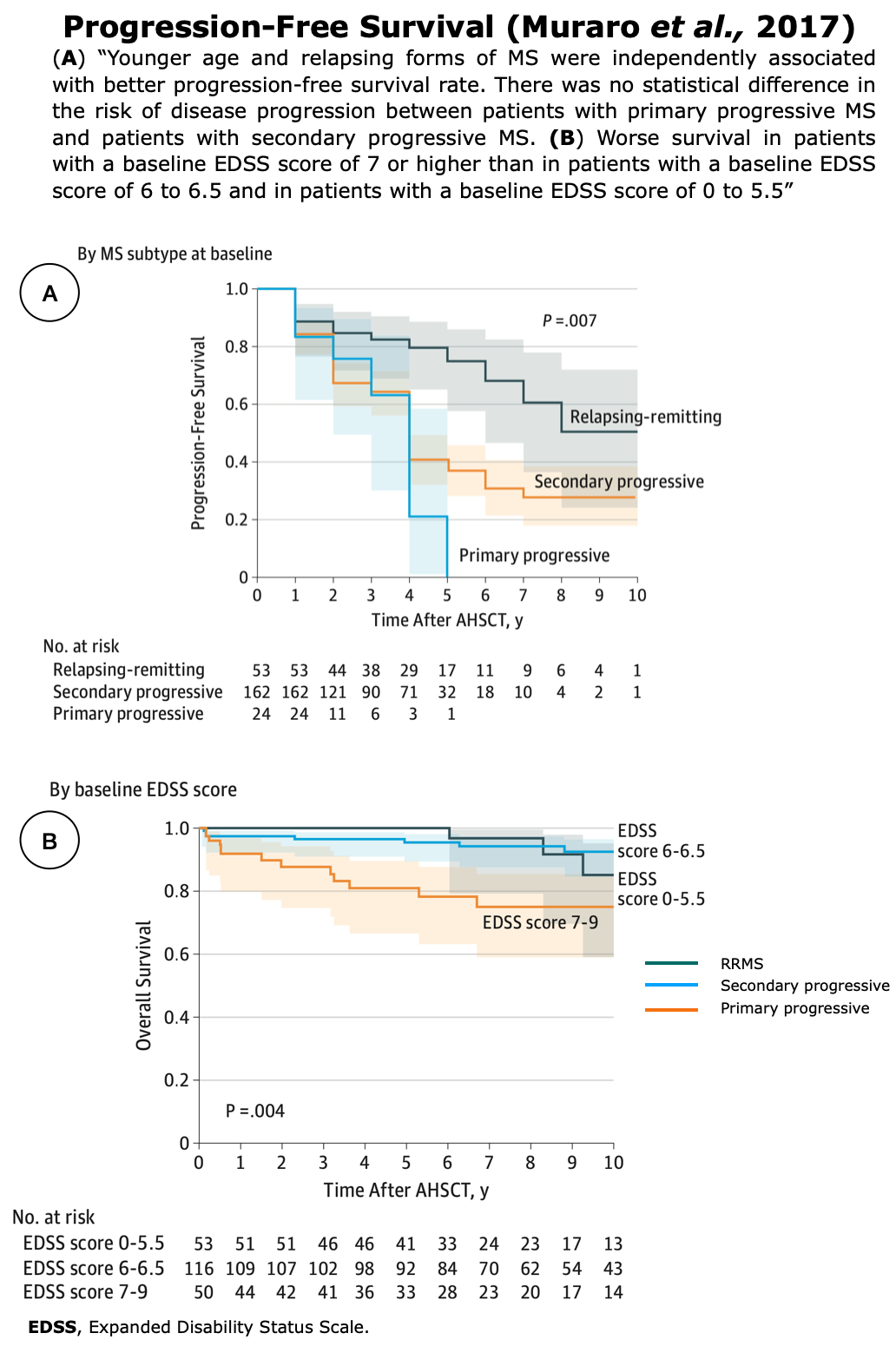
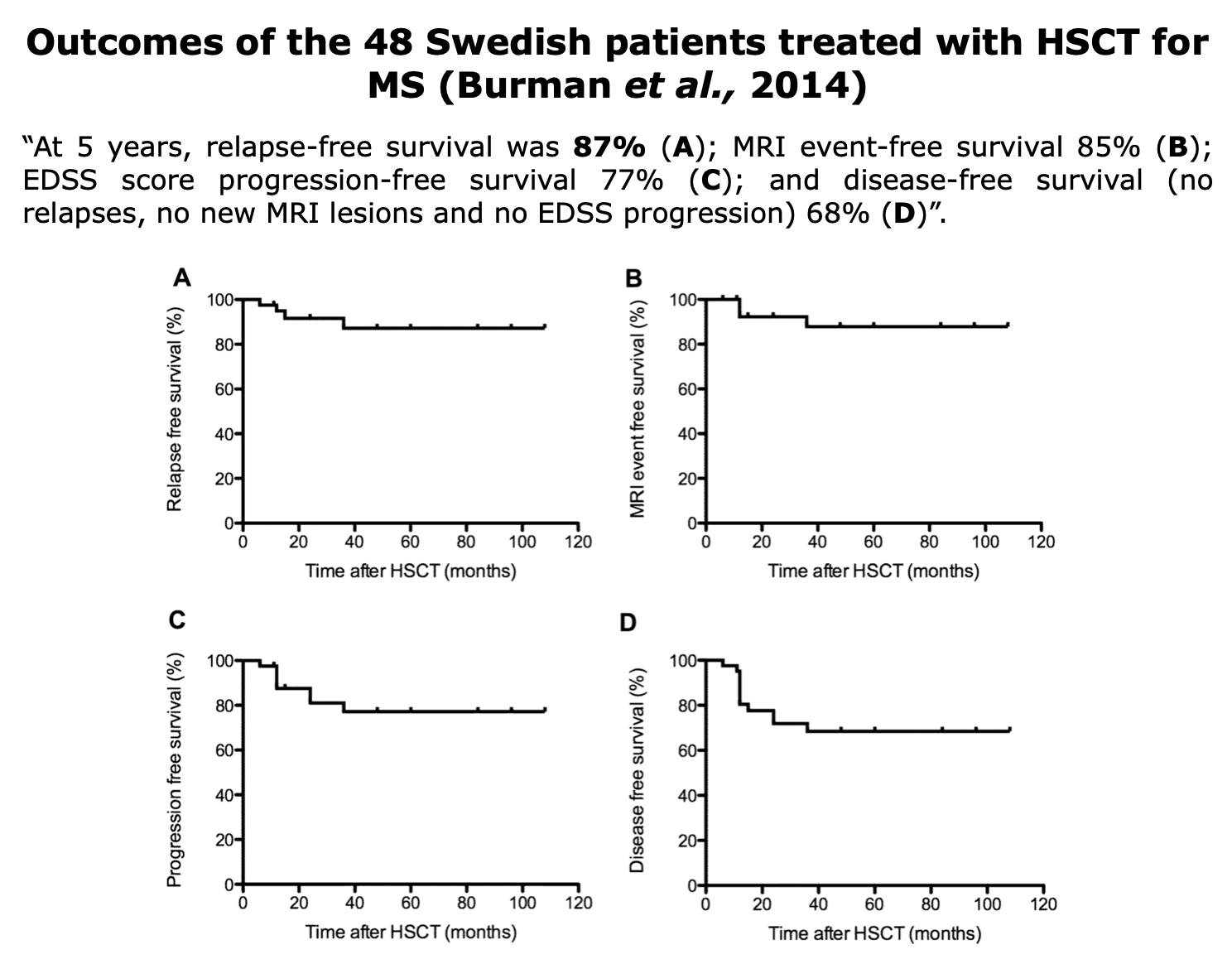
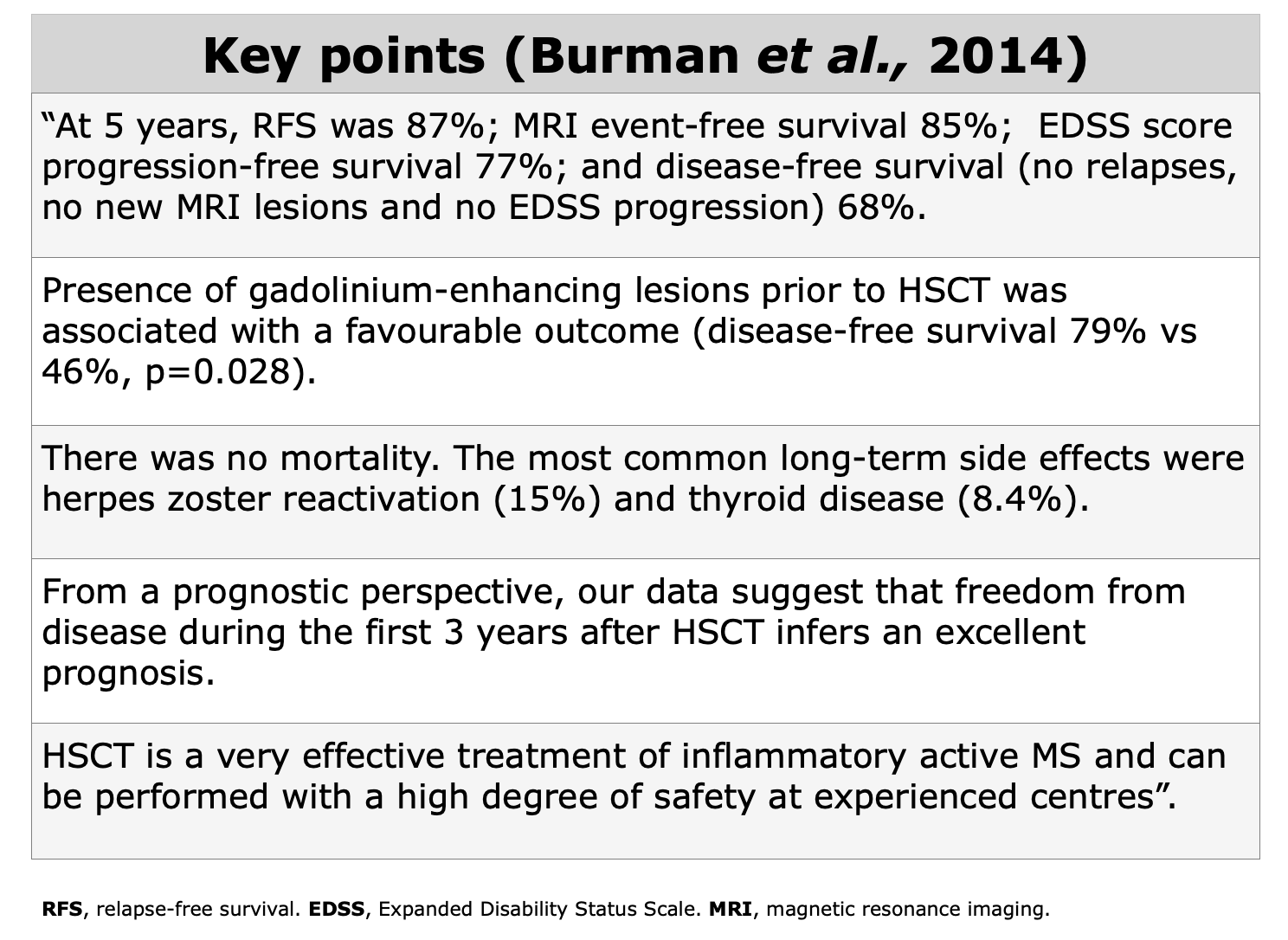
Clinical Evidences
Tools
Clinical evidences are information and data collected through scientific studies and clinical observations that demonstrate the efficacy, safety, and effectiveness of treatments or procedures.
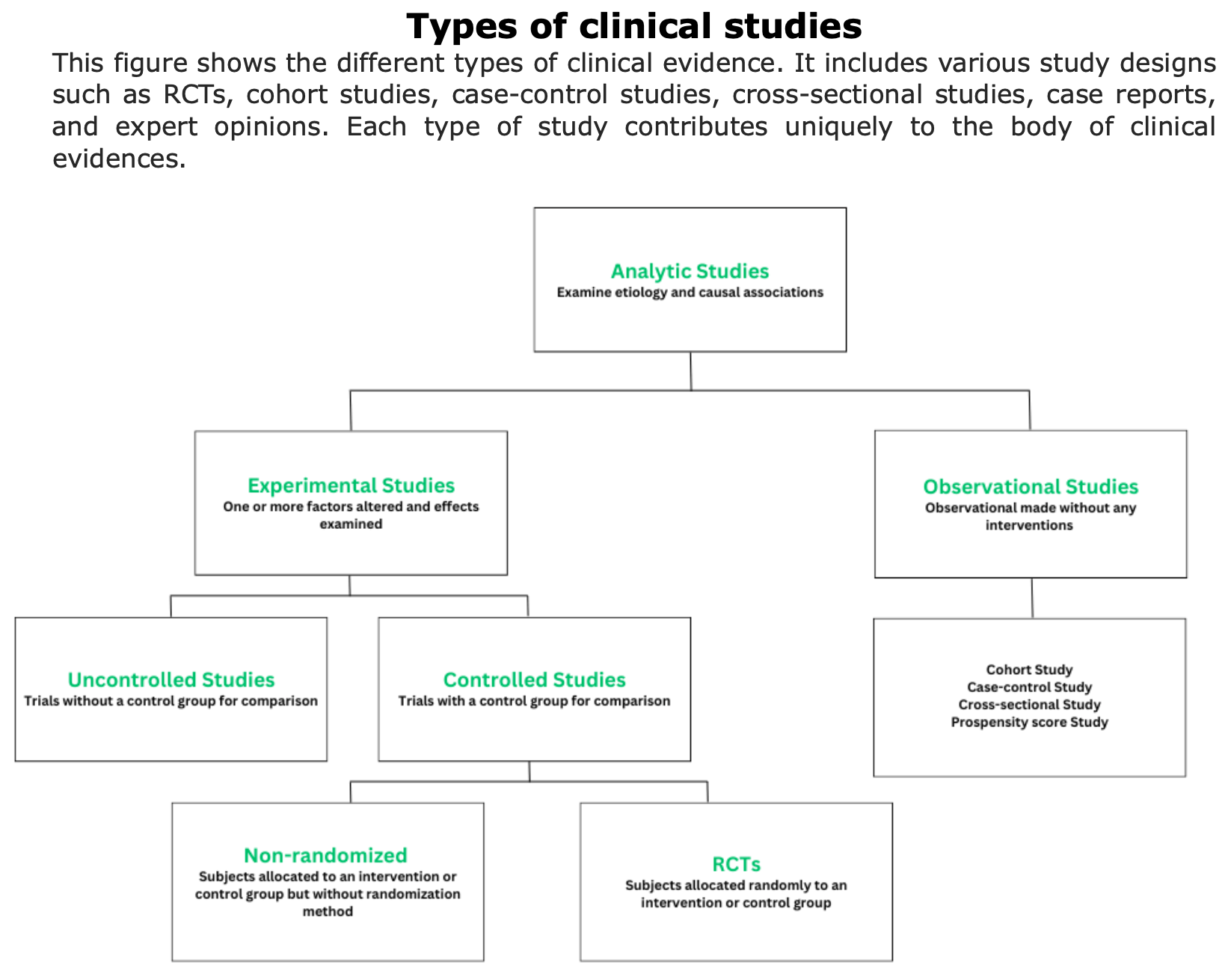
To obtain clinical evidences, various types of studies are conducted (e.g., randomized controlled trials, cohort studies, case-control studies, etc.). Each of these studies provides evidence of different strengths.
Click on these links for additional informational about how clinical evidences are collected, phases of clinical trials or class of evidences (Source: Neurology).
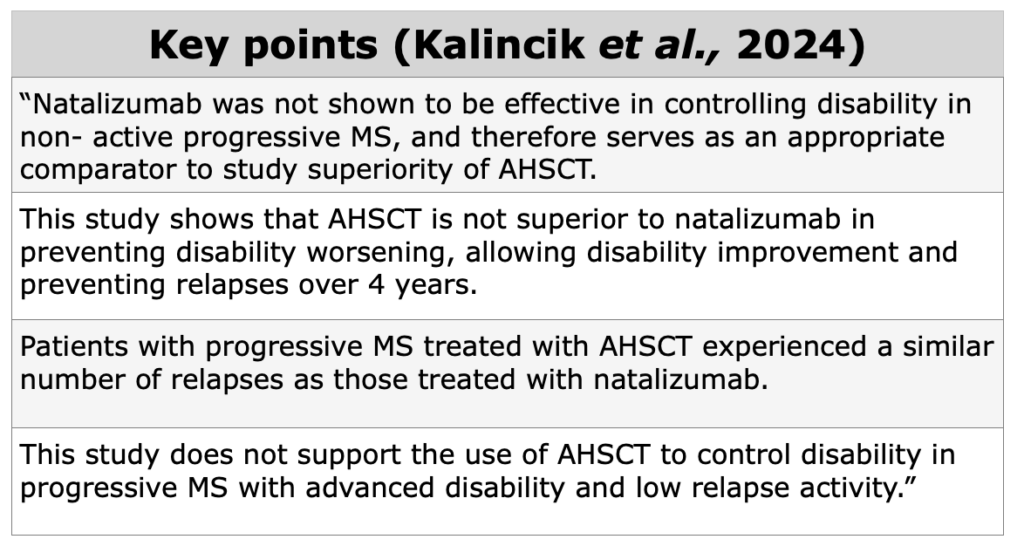
What are the indicators used to measure clinical evidence in MS?
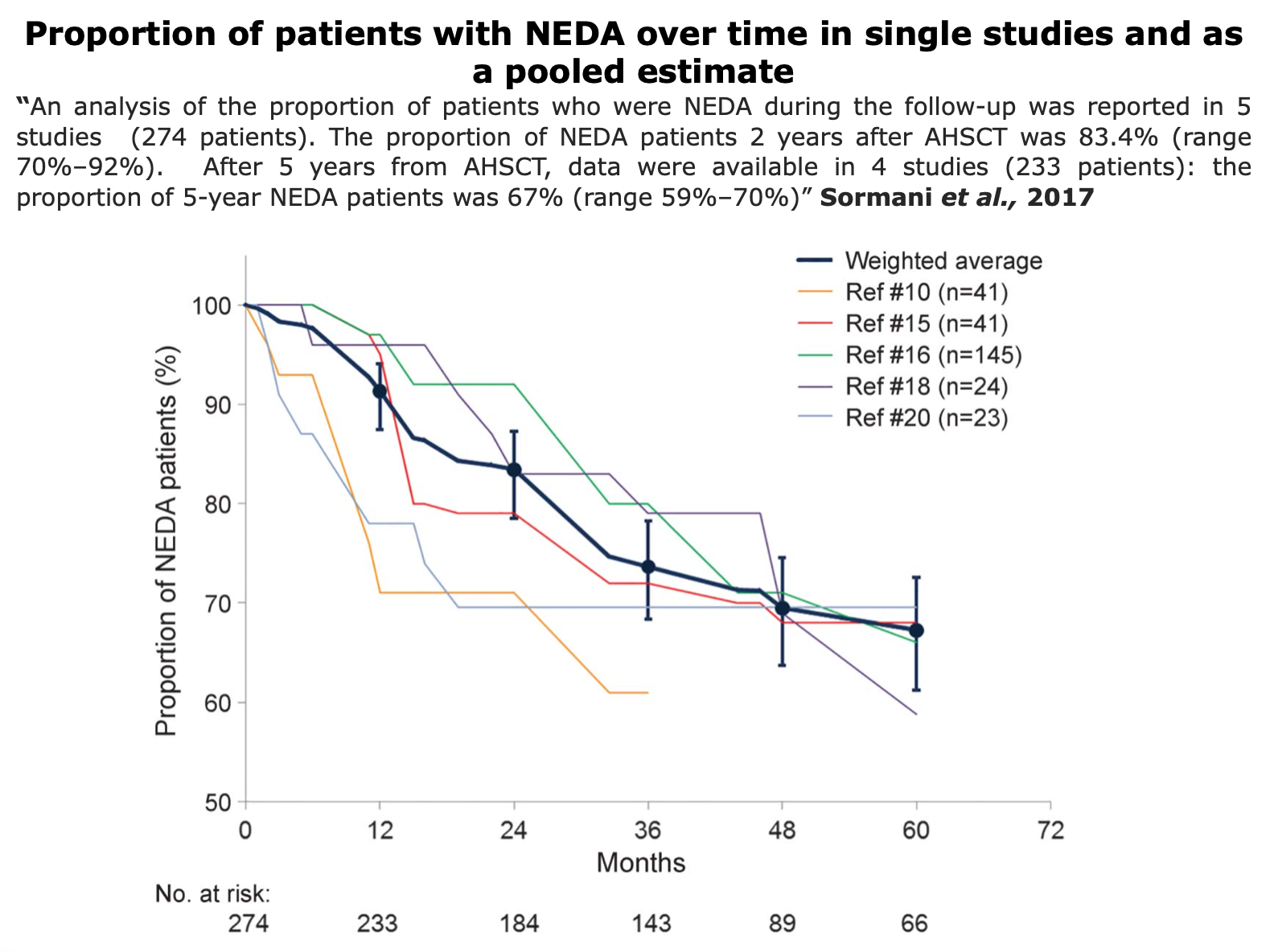
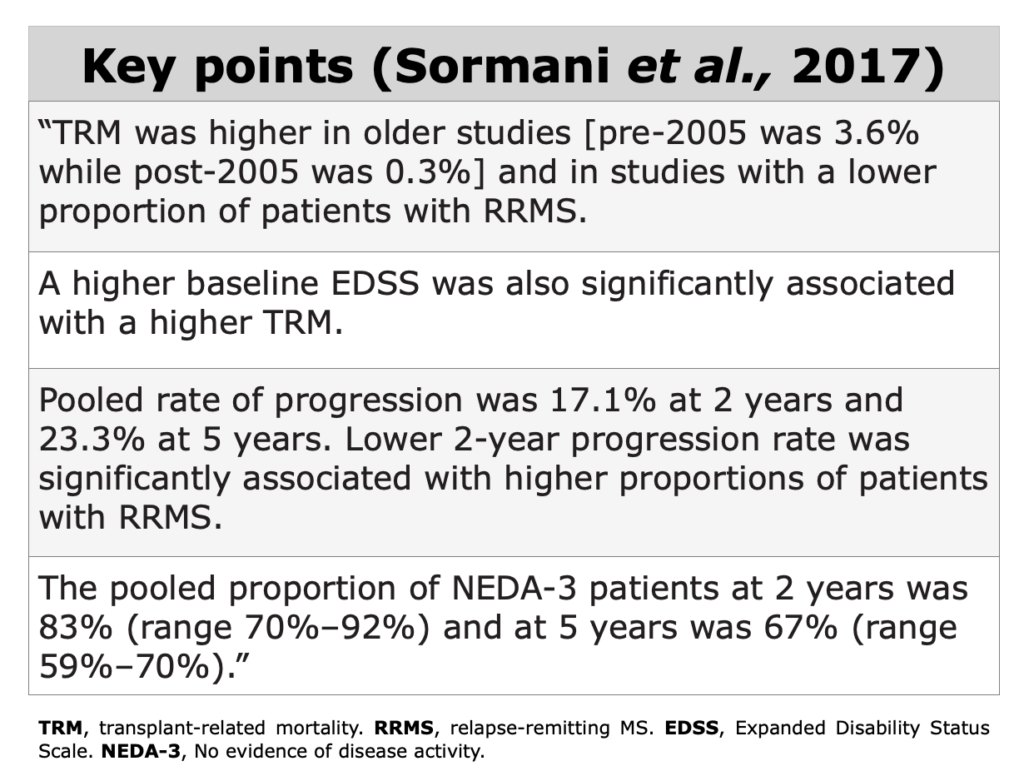
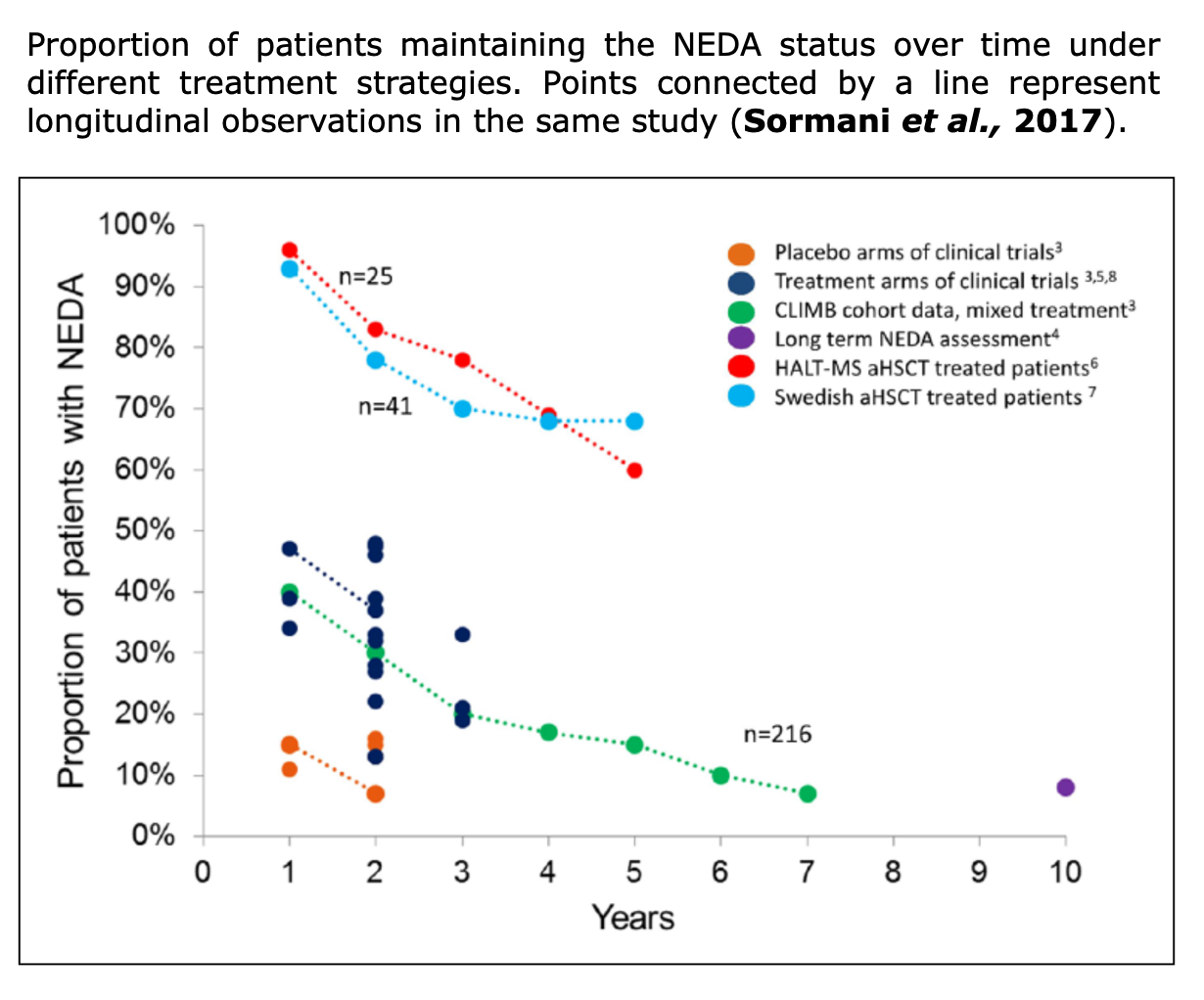
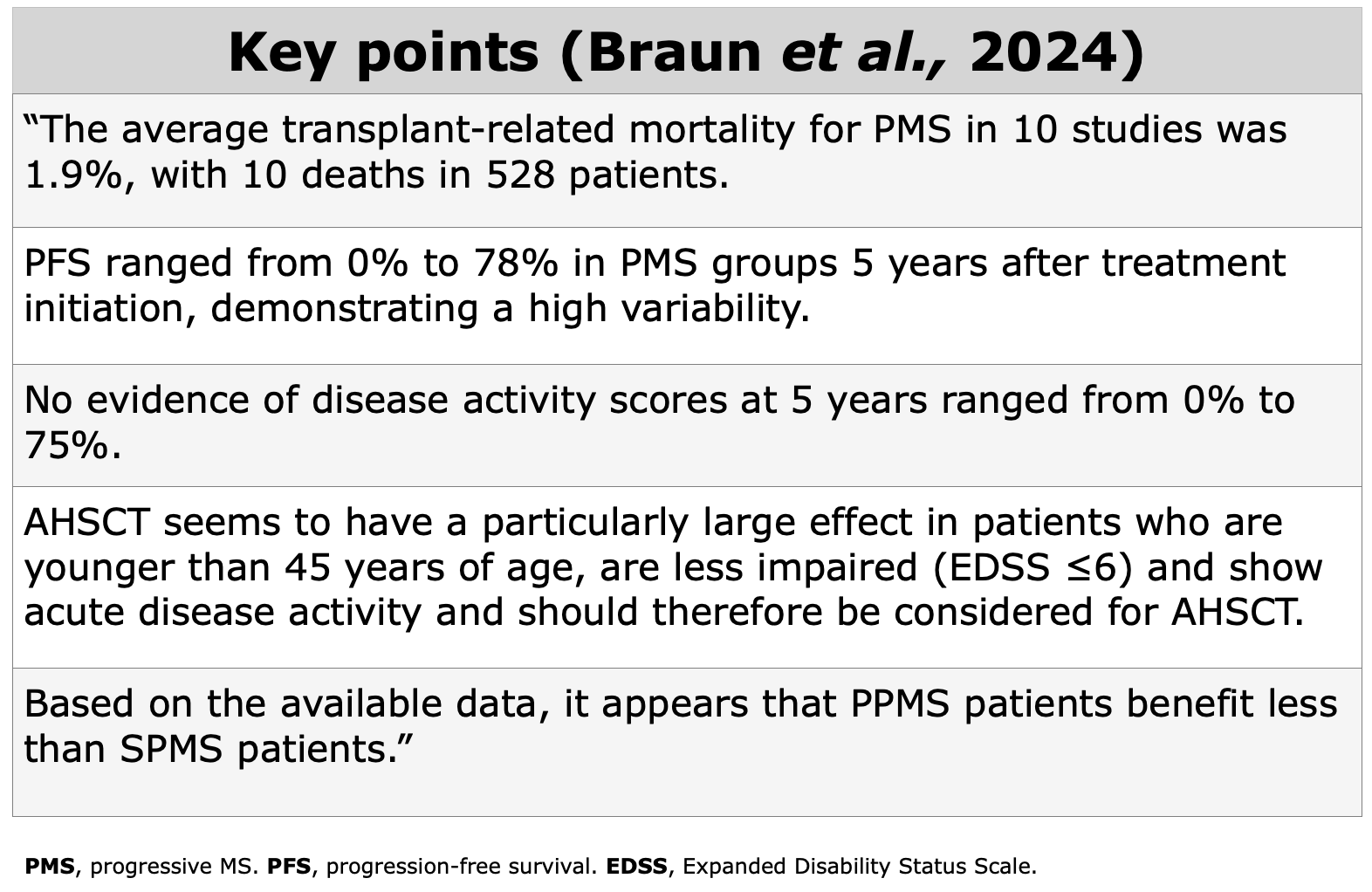
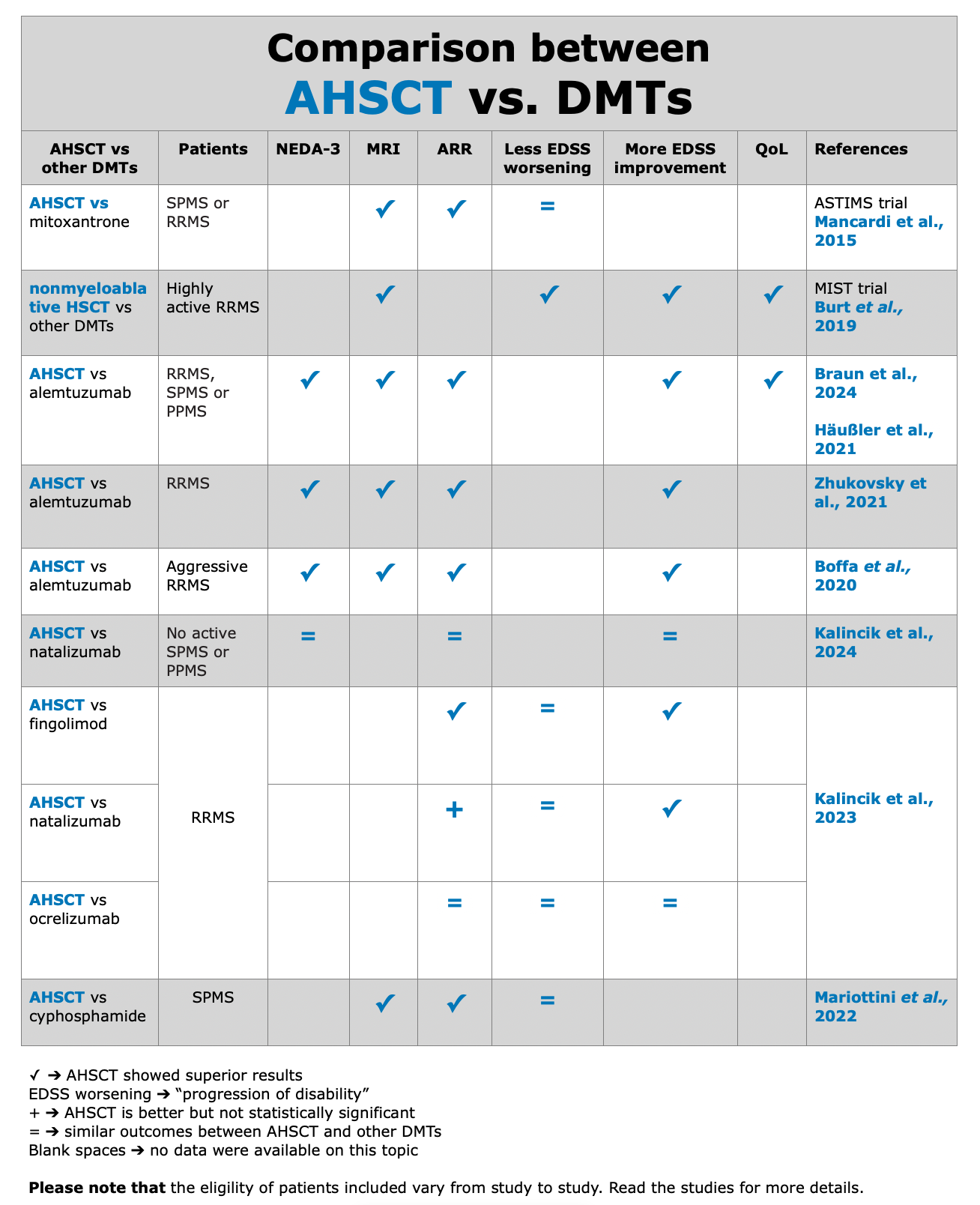
To assess clinical evidences in MS field, NEDA-3, relapse-rate, EDSS score, QoL, and radiological data as valuable indicators.
RCTs
Definition according to “A Dictionary of Epidemiology” (Miguel Porta, 2014):
RCTs completed
with published data
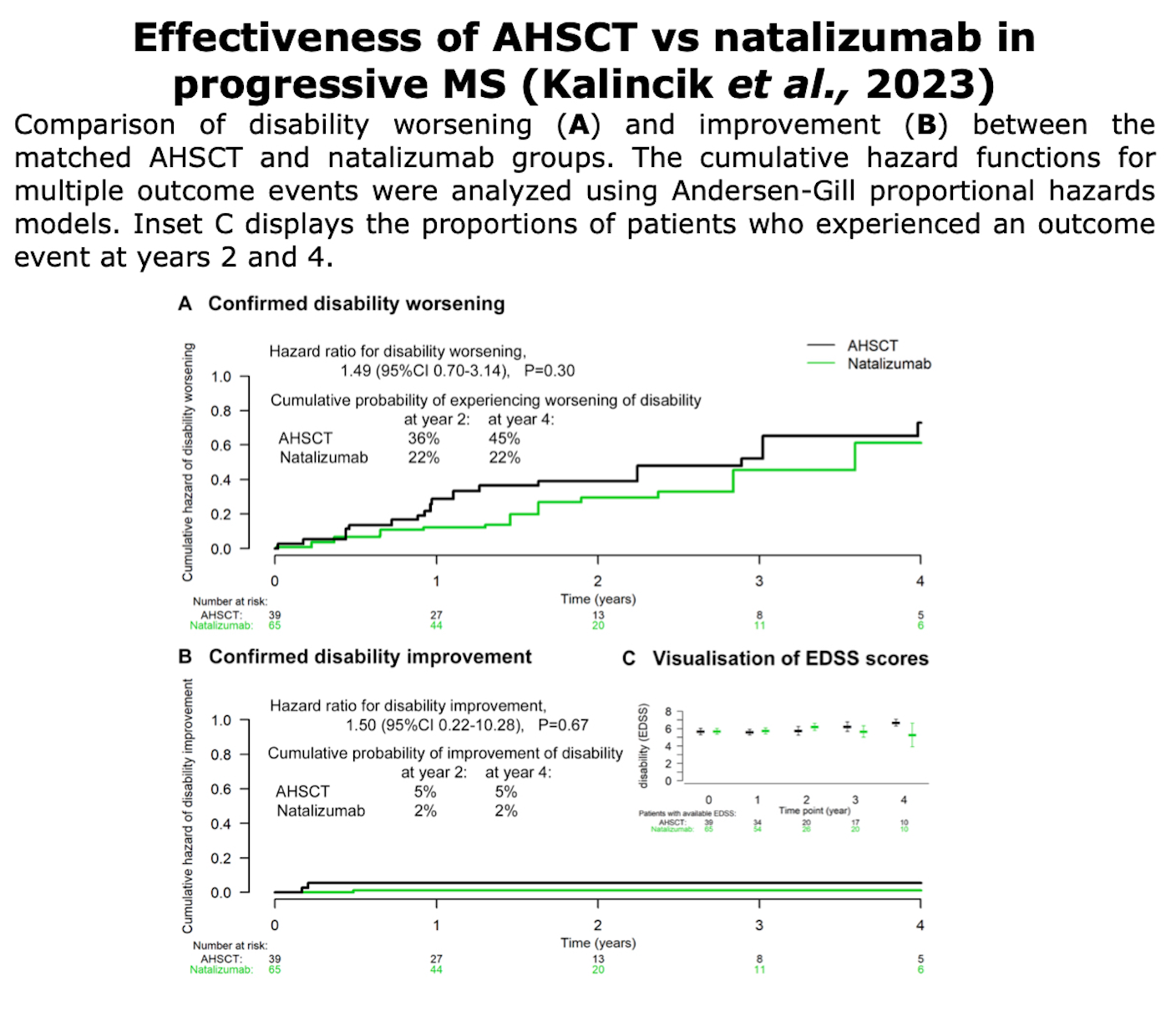
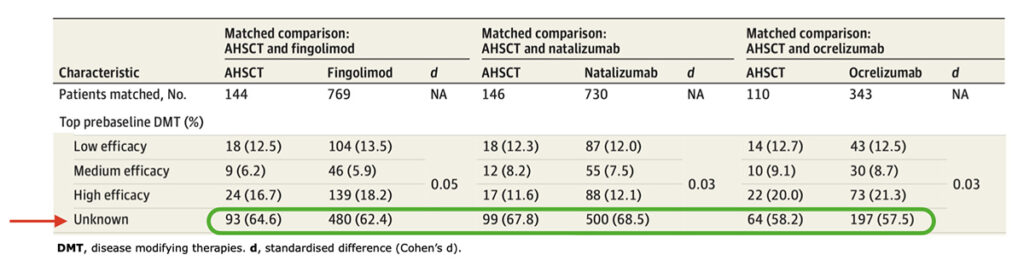
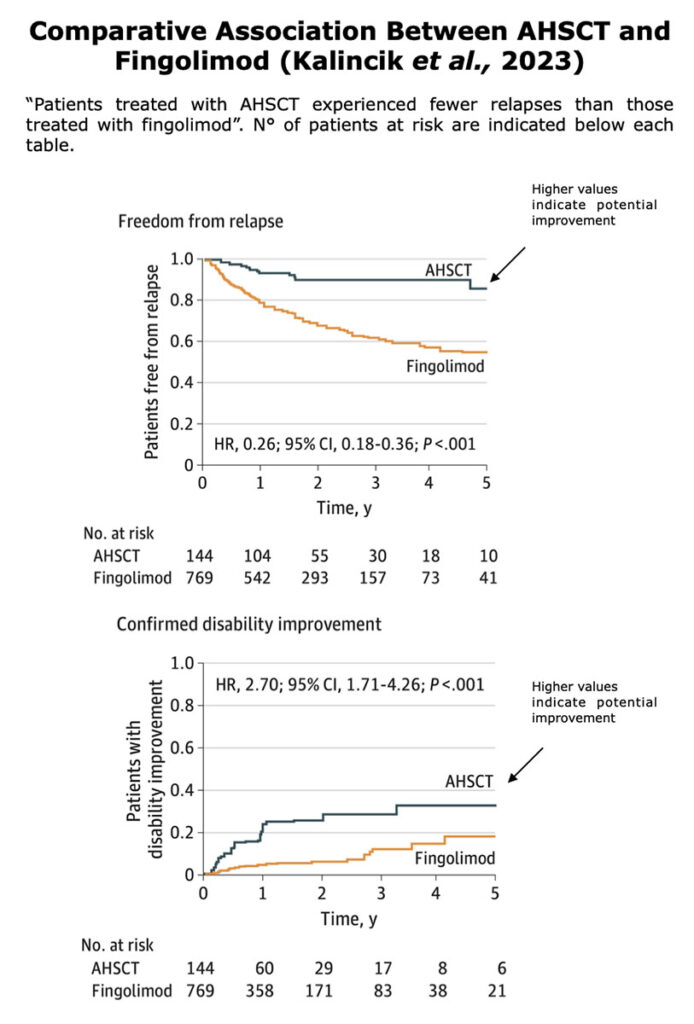
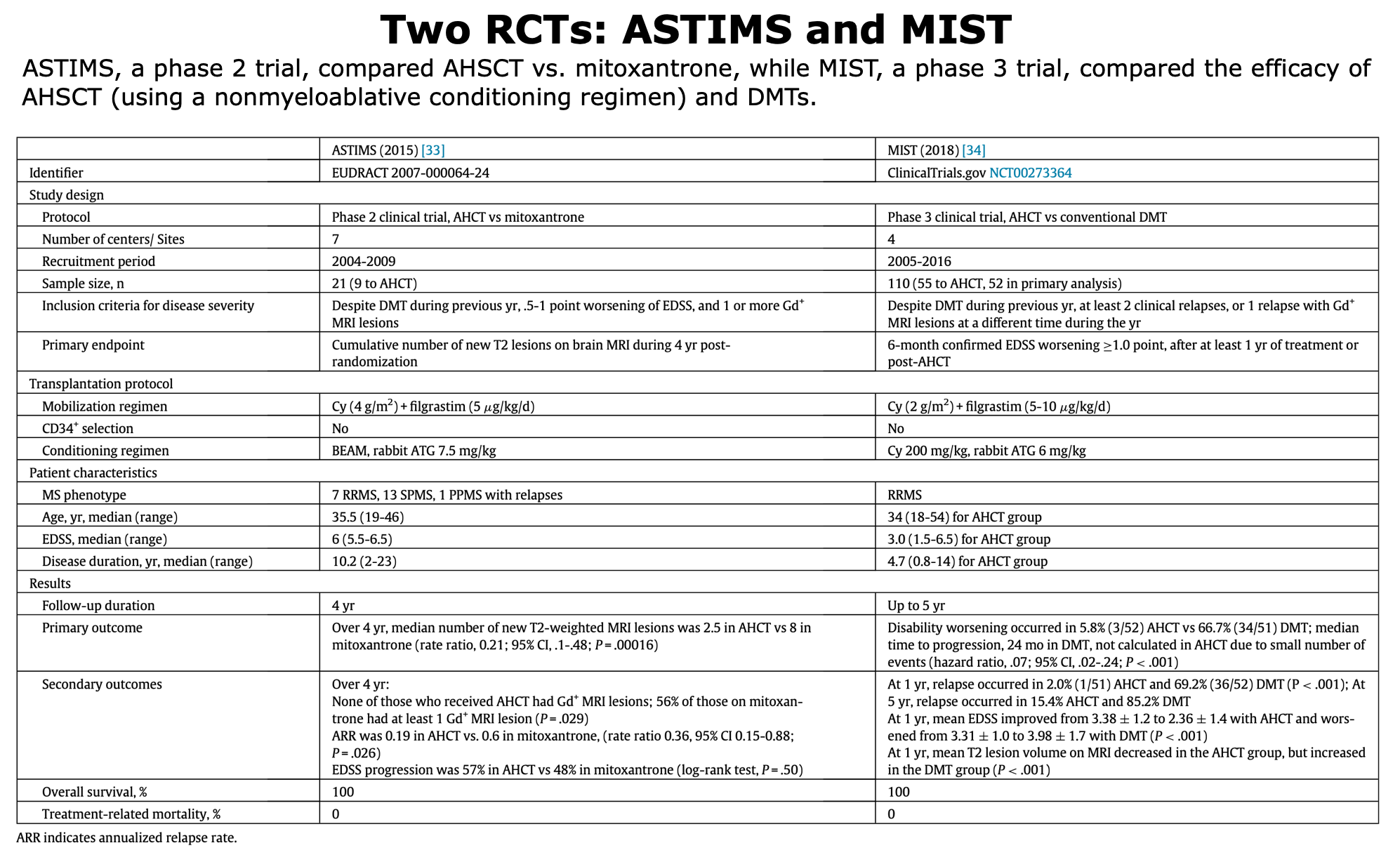
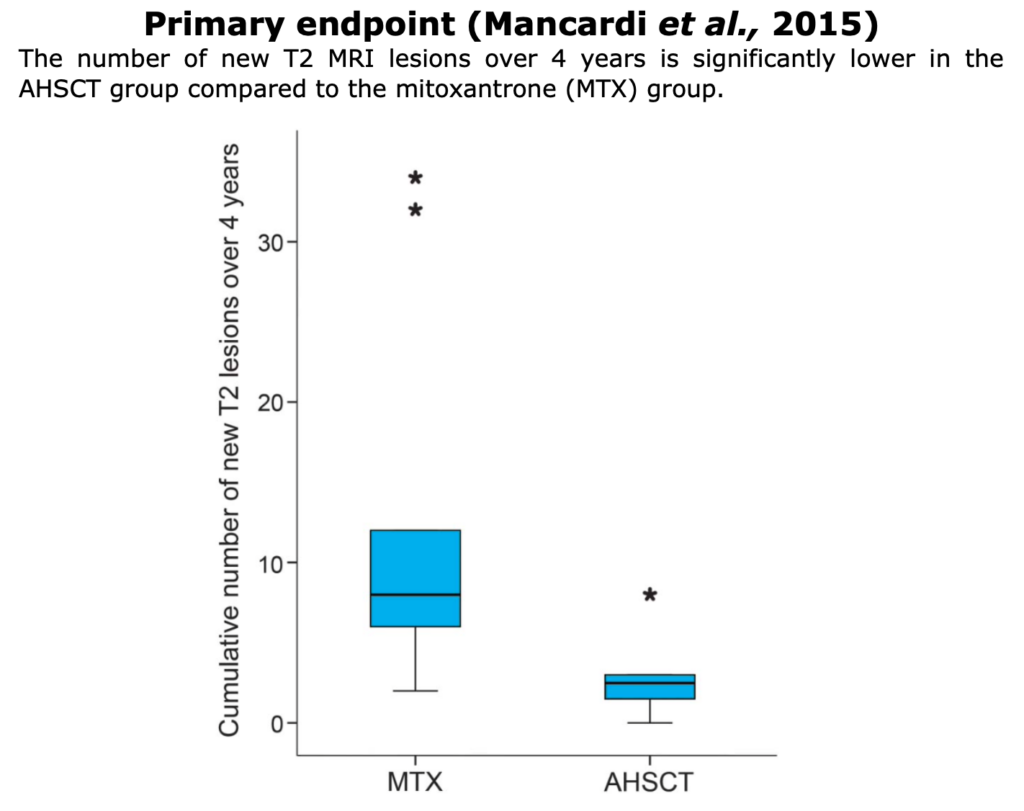
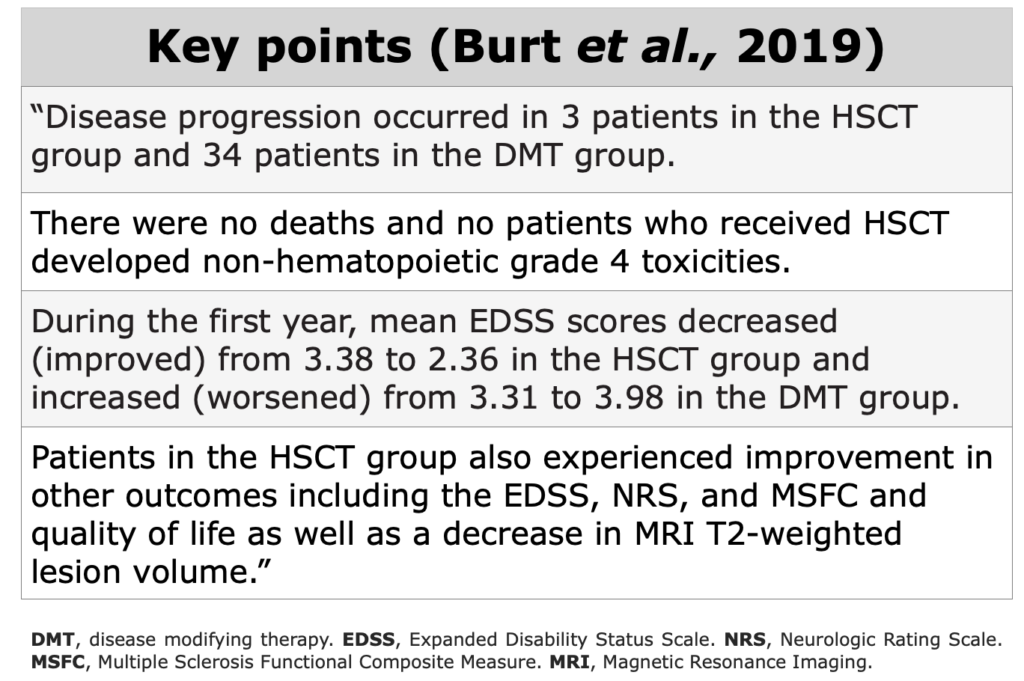
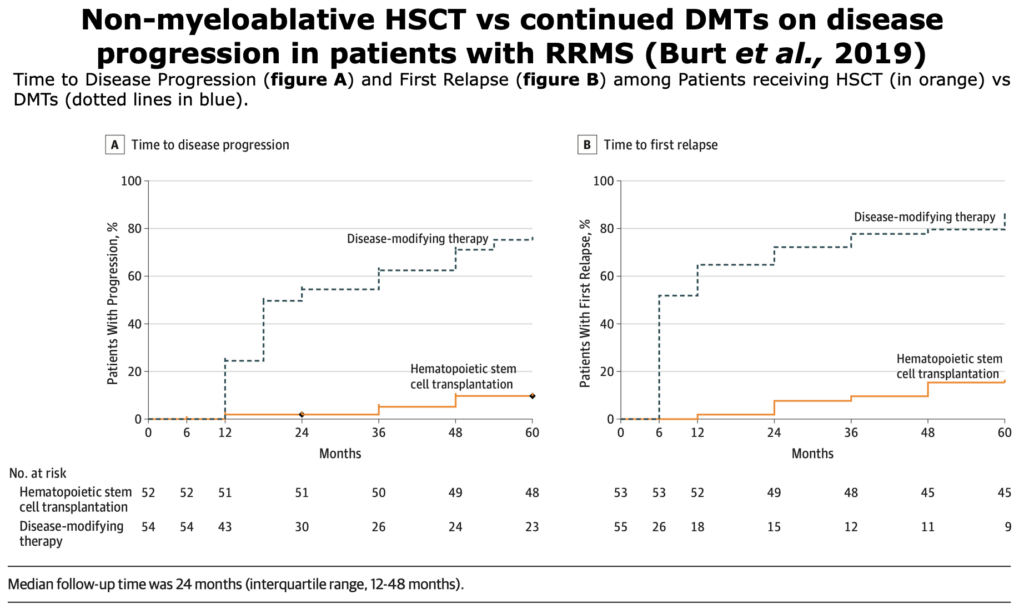
Below, the table that summarizes ASTIMS and MIST trial.
Table from Cohen et al. “Autologous Hematopoietic Cell Transplantation for Treatment-Refractory Relapsing Multiple Sclerosis: Position Statement from the American Society for Blood and Marrow Transplantation”. Biol Blood Marrow Transplant (2019).
RCTs - Ongoing Trials
To date, there are 4 ongoing trials. For more information, please visit the following links: BEAT-MS, NET-MS, RAM-MS trial and STAR-MS.
For the section dedicated to trials on this website, click here and here for an overview.
RCTs - Terminated
In the course of scientific research, the publication of trial results is a crucial step in advancing our knowledge. However, not all trials reach the publication stage due to challenges in recruiting, data collection, unexpected outcomes, methodological issues, or shifts in research focus.
Here we present two trials that were not published. The COAST TRIAL was terminated due to lack of recruitment caused by low acceptance of the control arm, while the MOST trial was terminated due to sabbatical principal investigator.
Observational Studies
In observational studies, researchers do not assign participants to get an intervention. If there is an intervention, participants were already using it as part of their regular health care or daily life”. The main limitation in these types of studies is that they are not randomized.
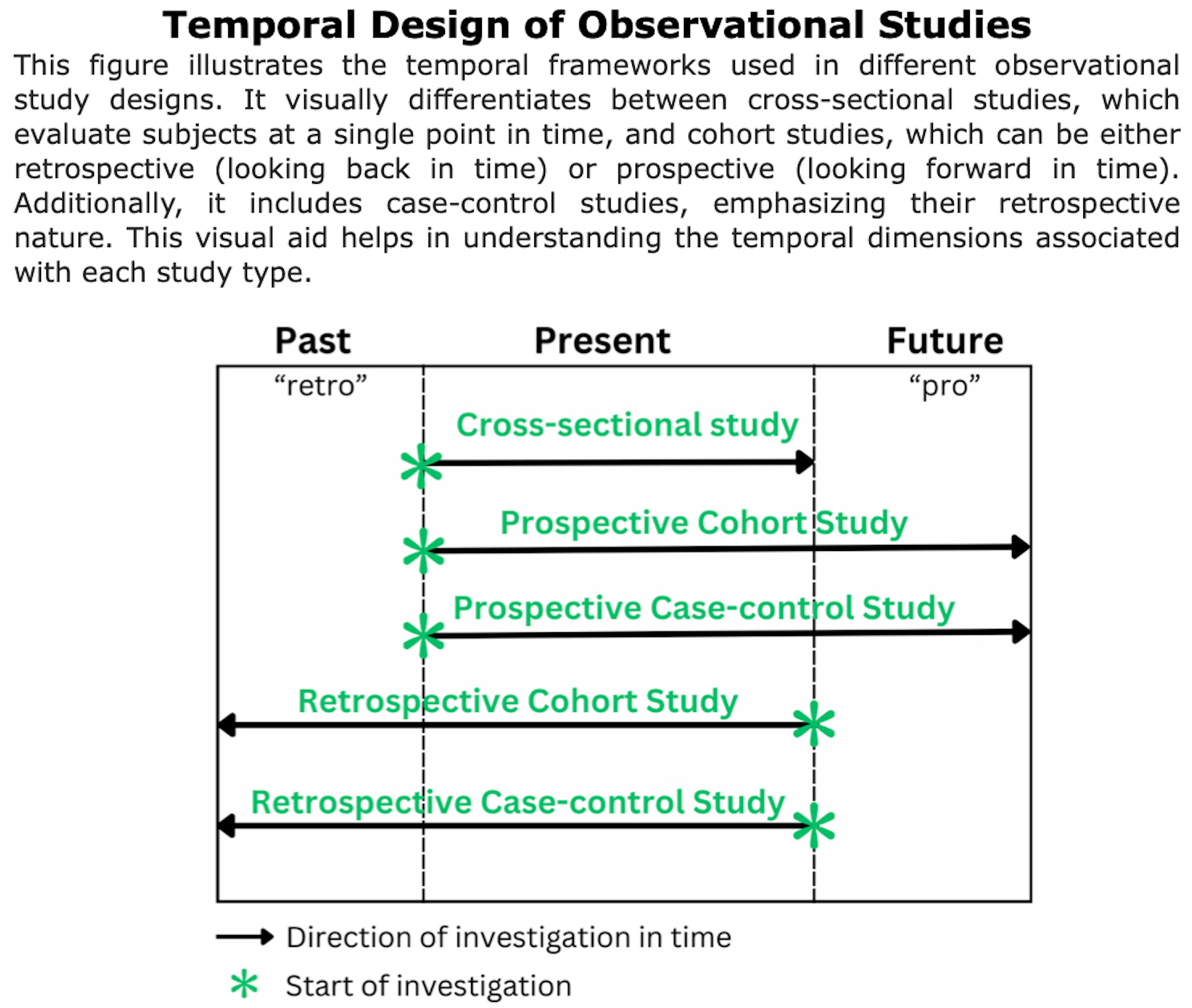
Observational Prospective Studies
Observational prospective studies follow a group of individuals over time to track their exposure to certain factors and health outcomes. Researchers analyze the relationship between exposures and outcomes to identify potential health risks.
Observational Retrospective Studies
Definition of retrospective studies: “Retrospective cohort studies, also known as historical cohort studies, are carried out at the present time and look to the past to examine medical events or outcomes. In other words, a cohort of subjects selected based on exposure status is chosen at the present time, and outcome data (i.e. disease status, event status), which was measured in the past, are reconstructed for analysis.” (Song et al., 2010).
These studies are “observational” because the researchers do not intervene or alter the course of events; instead, they analyze past data to find correlations or outcomes.
Propensity Score Studies
Retrospective studies with propensity scores are observational studies that use statistical techniques to reduce bias and simulate some of the characteristics of RCTs.
Case-Series
Meta-Analysis
While clinical trials produce new experimental evidence, meta-analyses summarize and integrate existing evidence.
Meta-analysis has a qualitative component (i.e., classification of studies according to predetermined characteristics capable of influencing results, such as study design, completeness and quality of data, absence of biases), and a quantitative component (i.e., extraction and analysis of the numerical information).”
Expert Opinion
Systemic Review
According to “A Dictionary of Epidemiology” (Miguel Porta, 2014), a systemic review “focus on peer-reviewed publications about a specific health problem and use rigorous, standardized methods for selecting and assessing articles. A systematic review differs from a meta-analysis in not including a quantitative summary of the results.”
Immunological Evidences
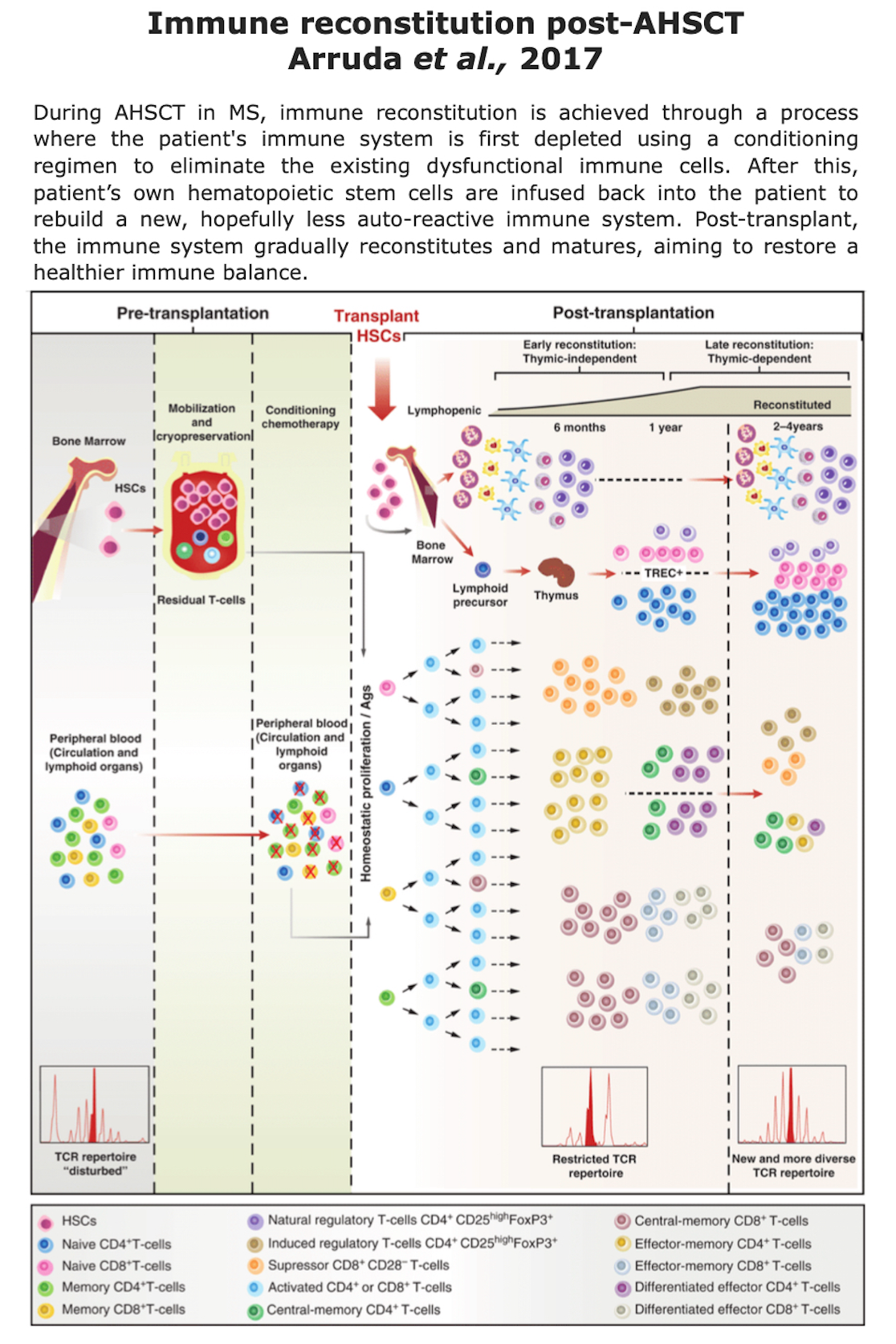
How Does AHSCT Act on the Immune System?
Clinical data suggest that the immune reconstitution (IR) following transplant is associated with sustained therapeutic benefits (read “Clinical evidences” section). The precise mechanisms by which AHSCT provides therapeutic effects remain unclear (Massey et al., 2018), however immunological data indicates that AHSCT in MS involves a reset of the immune system through:
- Ablation (destruction) of pathogenic autoreactive immune cells
- Immune reconstitution from myeloid or lymphoid progenitor cells
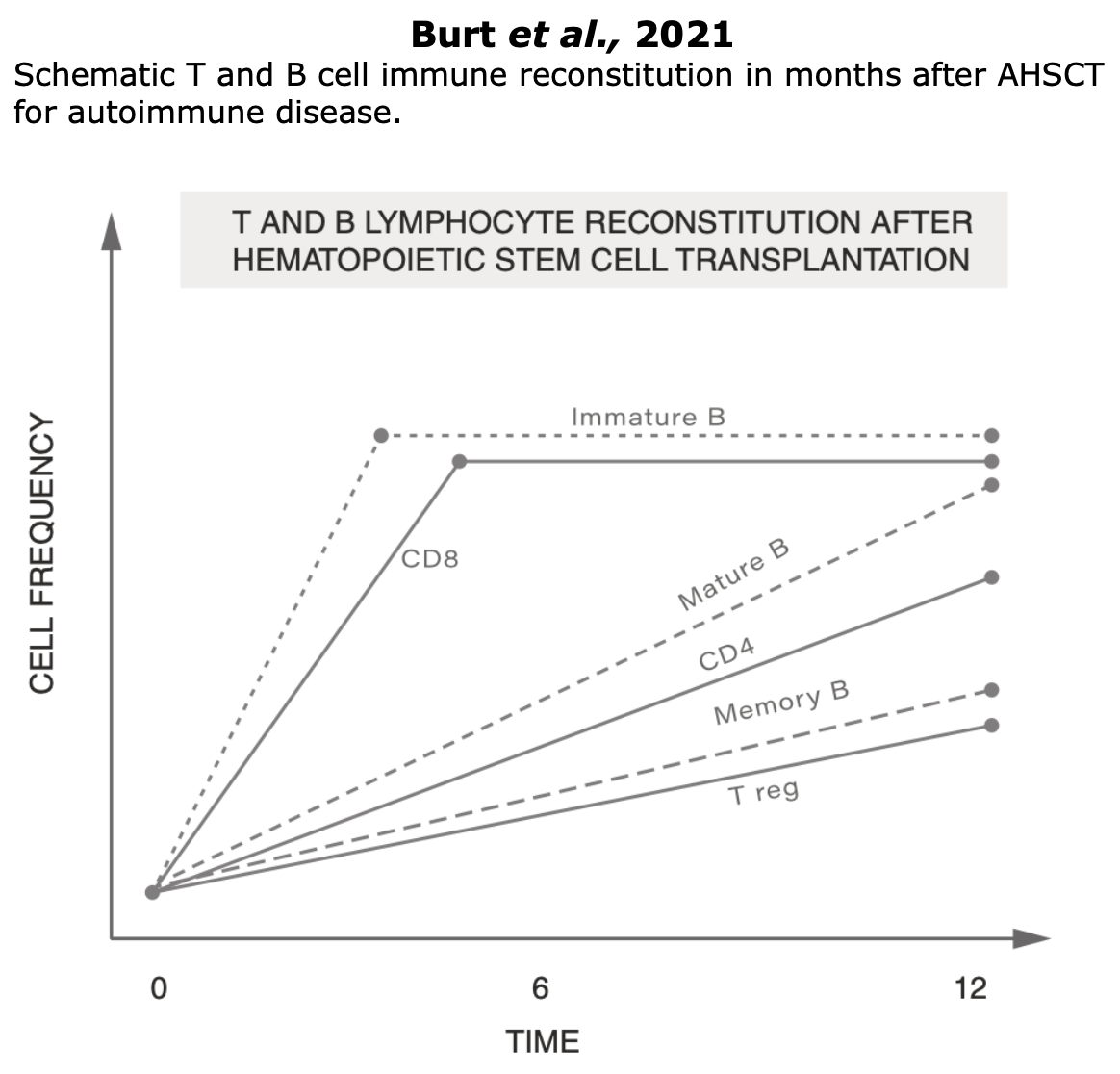
- Fast recovery of innate immunity cells (neutrophils, macrophages, and NK cells), which return to baseline levels (few weeks after AHSCT)
- Slow recovery of adaptative immune cells (3-6 months)
- Slower recovery in regulatory cell levels (6-12 months)
- Normalization of immunological functions.
Additional insights can be found in the reviews Arruda et al., 2017, Cencioni et al., 2021 and Mariottini et al., 2023.
Figure from Arruda et al. “Section 15 Hematopoietic Cell Transplants for Non-Neoplastic Diseases. Autologous Hematopoietic Cell Transplants for Autoimmune Diseases: Specific Diseases and Controversies”. Cambridge University Press (2017).
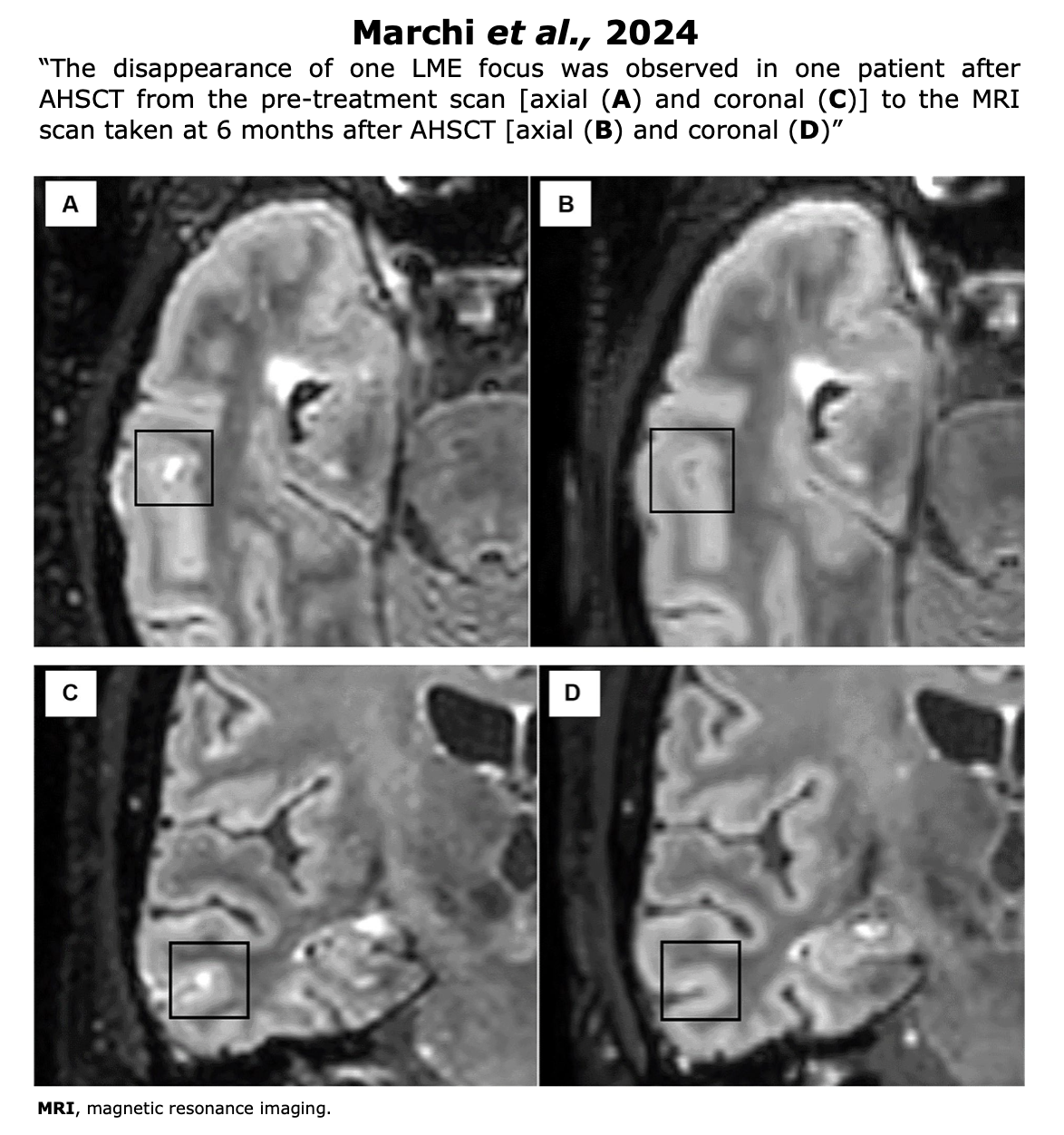
Papers on Immune Reconstitution Post-AHSCT
Below, some recent papers on the impact on immune reconstitution post-transplant. Click on the authors’ name to read the abstract or the full article where available. This section currently is in progress.
Quality Of Life
WHO defines Quality of Life (QoL) as “an individual’s perception of their position in life in the context of the culture and value systems in which they live and in relation to their goals, expectations, standards and concerns”.
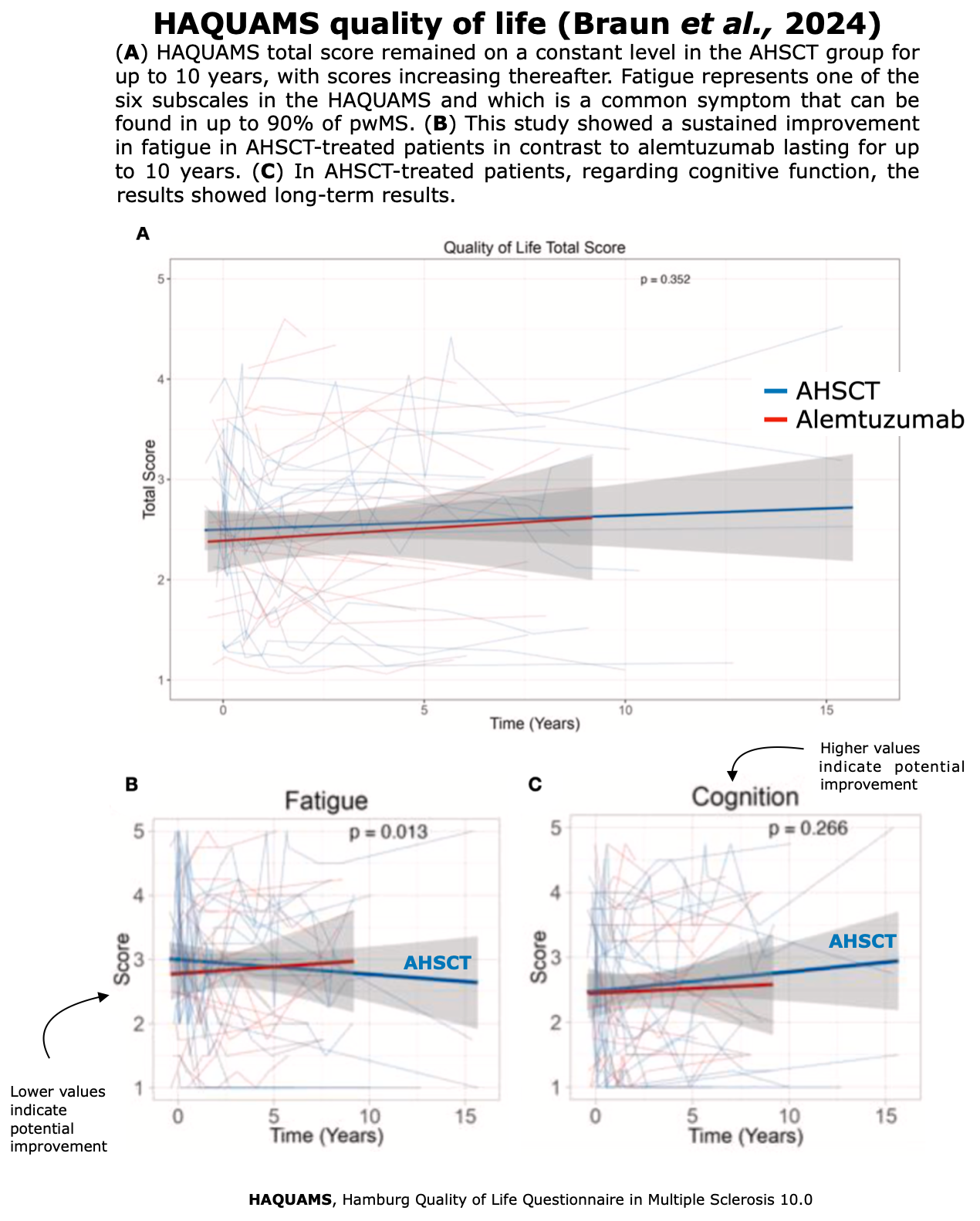
Improvements in health-related QoL have consistently correlated with sustained clinical stabilization (Muraro et al., 2025) and represent the most meaningful outcome for patients, as they directly impact their daily lives.
In pwMS, QoL is affected in numerous ways and aspects, including their ability to work, perform daily activities, and execute everyday tasks (Rezapour et al., 2017).
Initially, only the physical disability caused due to MS was considered by clinicians, as the sole aspect of the disease. In order to obtain an overview on how MS impairs patients’ QoL both psychological aspects and physical aspects must be considered.
All three of the studies listed below, published between 2022-2025, and focused on QoL following AHSCT, have demonstrated a sustained improvement in QoL.
QoL according to FDA: a patient-report outcome
QoL can be considered as a patient-reported outcome (PRO). PROs are defined by the US FDA as the “measurement of any aspect of a patient’s health status that comes directly from the patient, without the interpretation of the patient’s response by a clinician or anyone else” (US Food and Drug Administration 2009) (EBMT handbook – Barata et al., 2024). Consequently, PROs describe the impact that AHSCT has on patients’ lives.
As reported in the EBMT handbook (2024) there are numerous measures for assessing QoL. These measures include both general and disease-specific assessments. Trials like STAR-MS, RAM-MS and NET-MS have included QoL as a secondary outcome, emphasizing that incorporating QoL alongside other measures in clinical and research settings offers a more comprehensive understanding of AHSCT outcomes.
Pharmacoeconomics
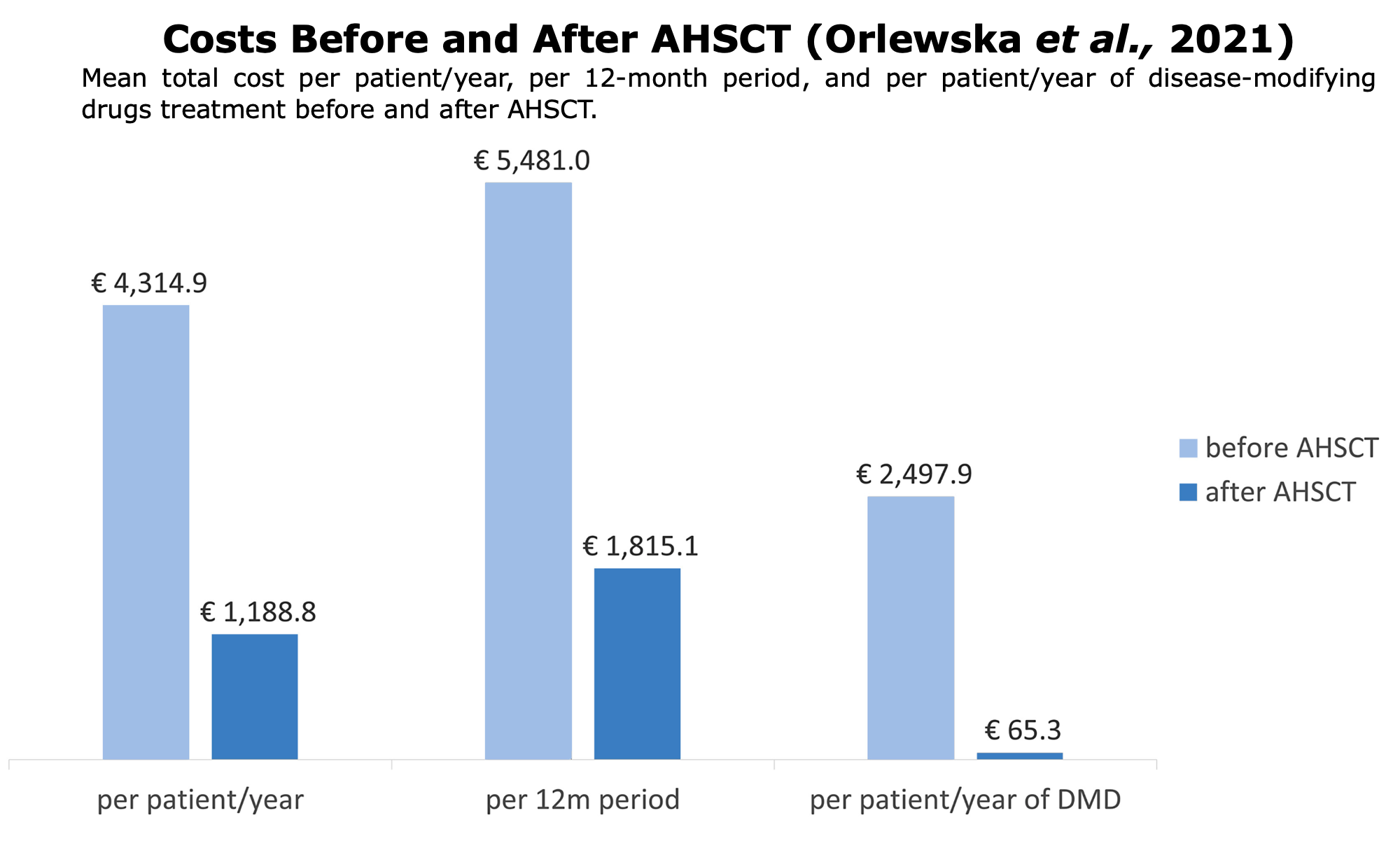
Pharmacoeconomics is a specialized branch of economics that assesses the cost and value of pharmaceutical products and services. It examines their cost and the benefits they provide in terms of reducing disease progression, disability, and improving patients’ quality of life (QoL).
Pharmacoeconomics employs various methods, such as cost-effectiveness analysis, to evaluate these aspects. These studies are essential for all healthcare systems, given the increasing challenges in providing medications to all citizens.
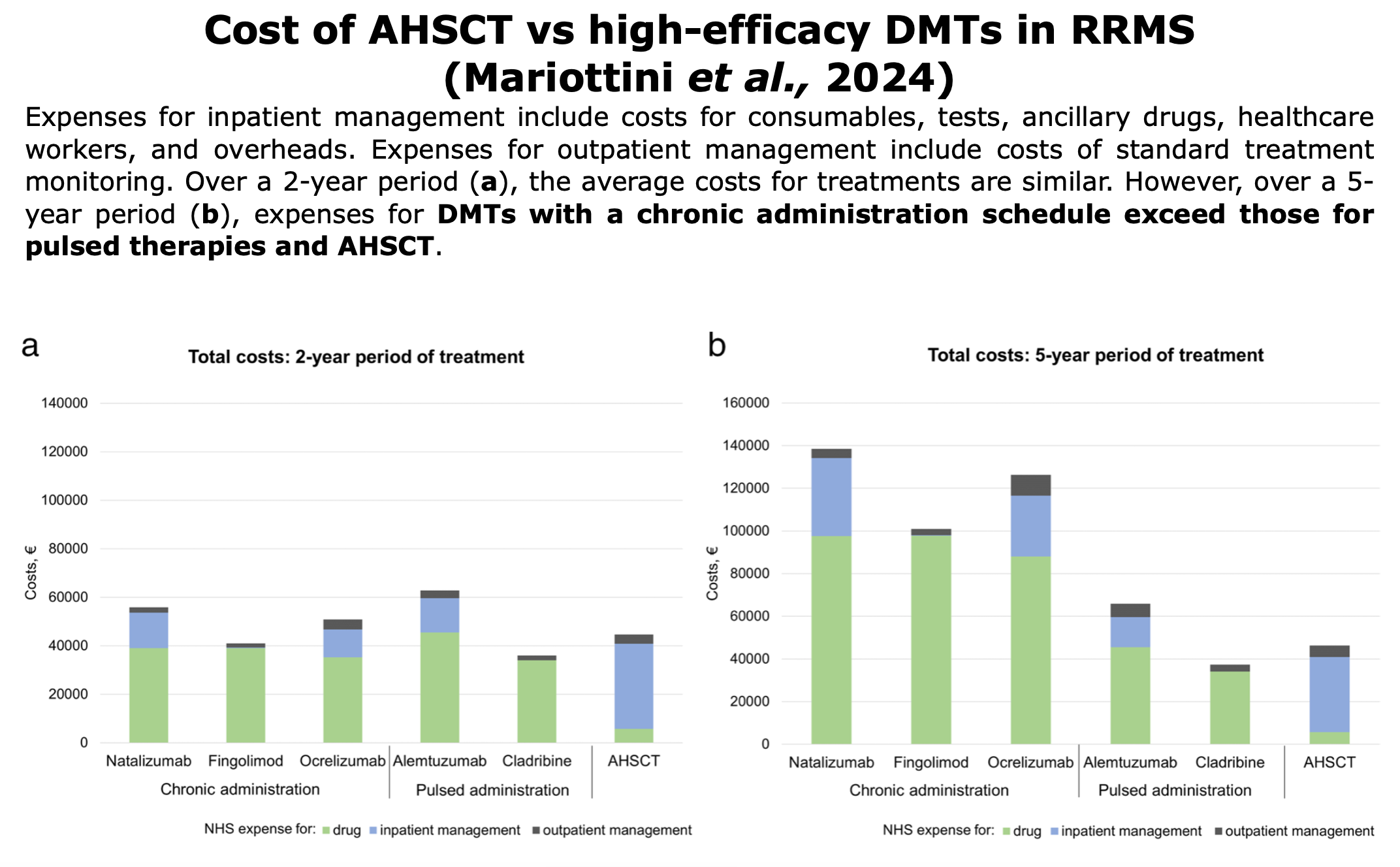
Pharmacoeconomic studies has consistently shown that transplants offer greater benefits to patients at a lower cost (“more effective, less costly“) than traditional DMTs. These findings apply to both public healthcare systems (such as in Italy, Norway, and Poland) and private ones.
Following studies or reports on pharmacoeconomic around the world comparing AHSCT vs. DMTs:


 Figures from
Figures from