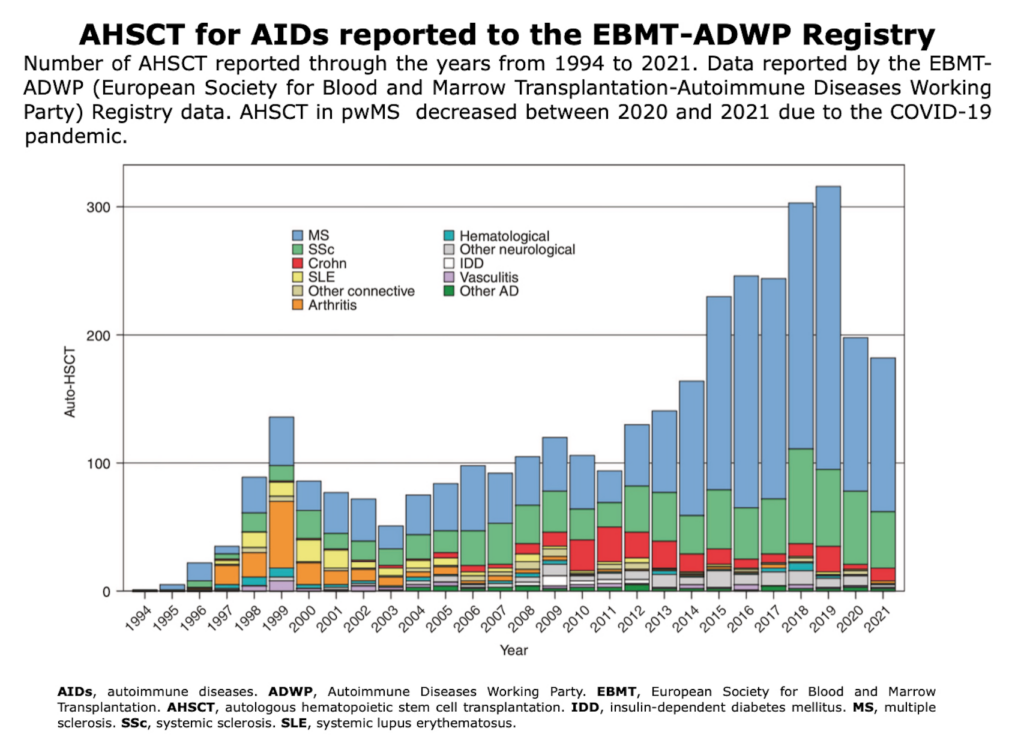
Overview
Initially designed for treating hematological cancers, over the past 50 years, this technique has been widely utilized to treat aggressive hematological cancers, including leukemia and lymphoma (Appelbaum FR, 2017). This procedure has been modified for use in severe immune-mediated disorders such as MS (Fassas et al., 1997) (Burt et al., 2015) (Muraro et al., 2017) (Burt et al., 2021).
AHSCT is a stem cell therapy that uses the patient’s own cells to replace those in the immune system that mistakenly attacks the CNS. Chemotherapy is employed to reset the immune system by destroying certain types of cells, followed by reinfusion of the stem cells to help rebuild a healthier immune response.
AHSCT is a validated, one-off treatment and a multistep procedure that requires a coordinated, multidisciplinary team.
Table from Muraro et al. “Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis and neuromyelitis optica spectrum disorder — recommendations from ECTRIMS and the EBMT“. Nature Rev Neurol (2025).
The entire procedure consists of three main phases: pre-AHSCT, AHSCT and post-AHSCT (i.e., prophylaxis and vaccinations):
Figure modified from Jessop et al. ”General information for patients and carers considering haematopoietic stem cell transplantation (HSCT) for severe autoimmune diseases (ADs): A position statement from the EBMT Autoimmune Diseases Working Party (ADWP), the EBMT Nurses Group, the EBMT Patient, Family and Donor Committee and the Joint Accreditation Committee of ISCT and EBMT (JACIE)” Bone Marrow Transplantation (2018).
Explore AHSCT in this brief video: learn how the procedure works and how it resets the immune system.
📌 For the full version of this video, click here.
AHSCT
During this phase, the patient’s own hematopoietic stem cells are collected and processed, followed by their reinfusion after targeted preparation. This process aims to restore the patient’s immune system, thereby reducing the autoimmune activity that characterizes MS.
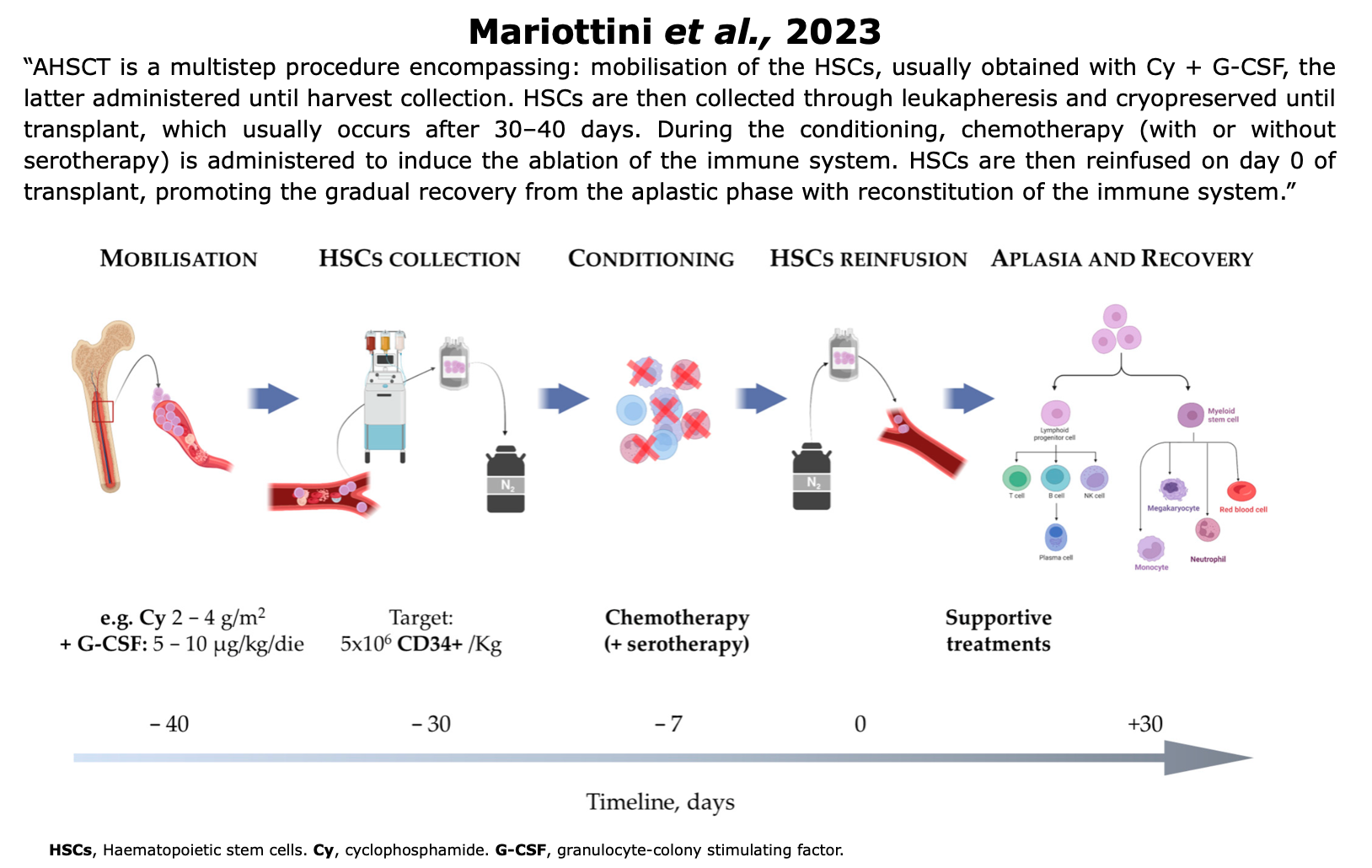
Mobilization
In the weeks or months leading up to the transplant, the patient undergoes a stem cell mobilization procedure to collect stem cells that will be used in the later transplantation process (Das et al., 2019, Roberts et al., 2020).
HSCs reside in the bone marrow. HSCs mobilization refers to the process of stimulating the release of stem cells from the bone marrow into the bloodstream, typically through the administration of granulocyte colony-stimulating factor (GSF), either alone or with cytotoxic chemotherapy, such as Cy (Muraro et al., 2017).
Figure from Mariottini et al. “Hematopoietic Stem Cell Transplantation for the Treatment of Autoimmune. Neurological Diseases: An Update“. Bioengineering (2023)
Harvest & Cryopreservation
Hematopoietic stem cells (HSCs) are now collected from peripheral blood through leukapheresis, a procedure that separates white blood cells from the bloodstream. In some cases, the graft is purified using CD34 immunomagnetic selection to remove mature immune cells.
After collection, HSCs are cryopreserved until transplantation. The recommended storage amount is 3–8 × 10⁶ CD34⁺ cells/kg, with an additional 2.5 × 10⁶ CD34⁺ cells/kg as a backup. A minimum of 3 × 10⁶ CD34⁺ cells/kg is required for reinfusion (Bayas et al., 2023).
Conditioning Regimen
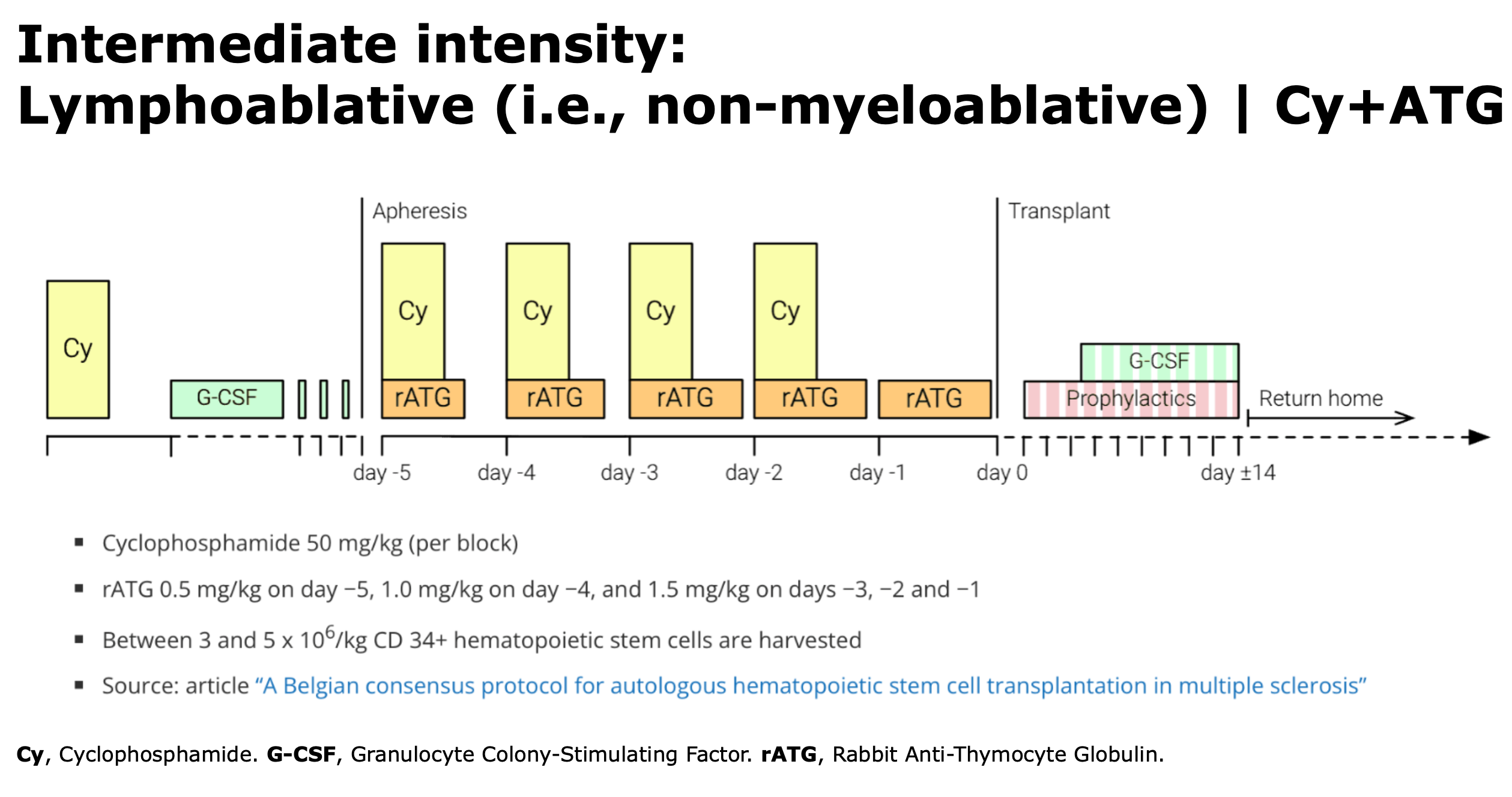
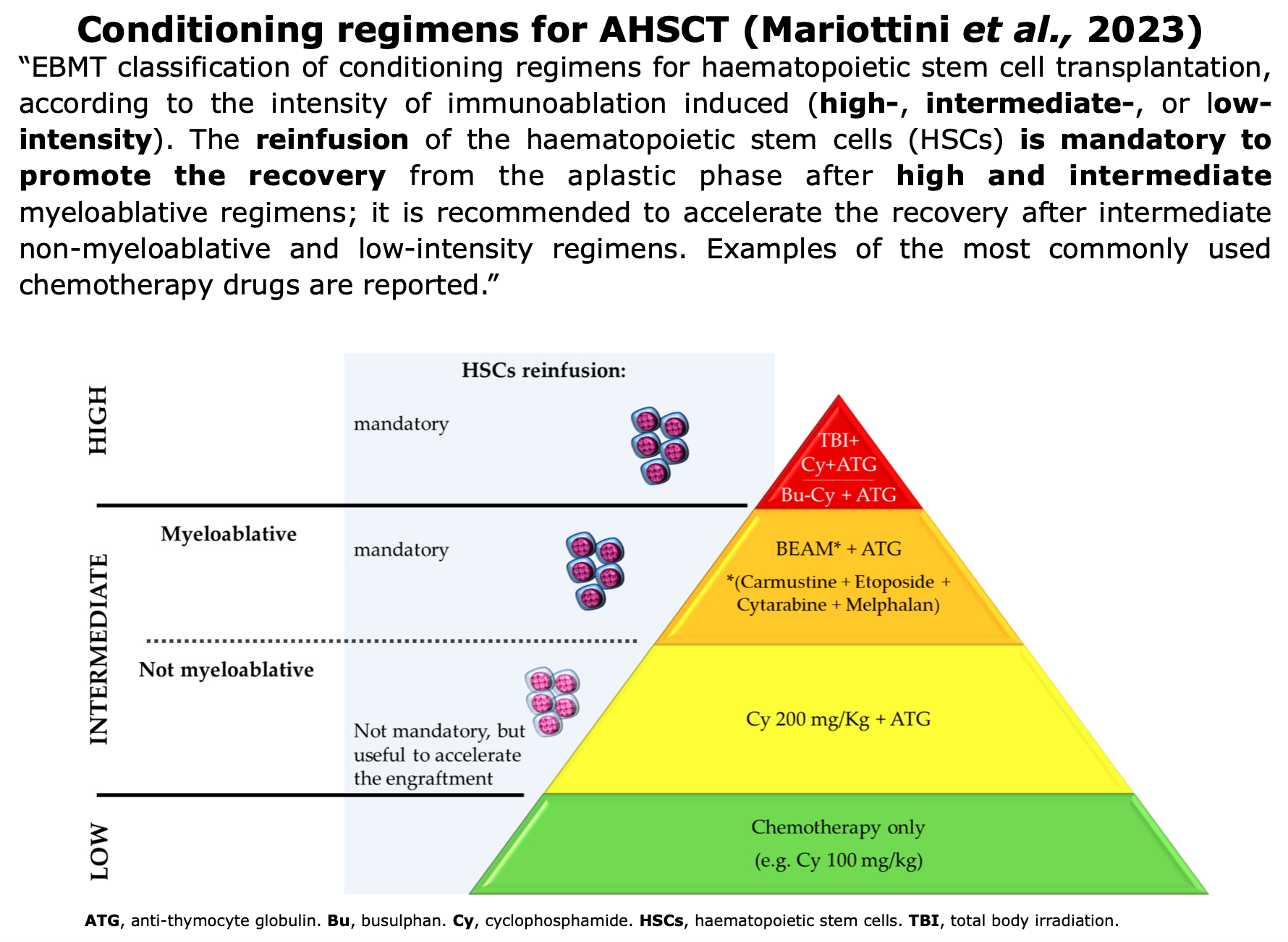
The goal is to eliminate existing immune cells. The transplant conditioning regimen start generally more than 3 weeks after mobilization. Various conditioning regimens are available and are classified according to the guidelines set by the EBMT.
Figure from Mariottini et al. ” Hematopoietic Stem Cell Transplantation for the Treatment of Autoimmune Neurological Diseases: An Update”. Bioengineering (2023)
Reinfusion
“Immediately after completion of the conditioning regimen, patients develop pancytopaenia and a transient bone marrow aplasia, and intravenous infusion of the stored HSCs (transplantation) is required to enable bone marrow repopulation, recovery of haematopoiesis, and immune reconstitution.” (Muraro et al., 2017).
This phase is considered as day “0” of the transplant. The patient’s own HSCs are reinfused, initiating the gradual recovery from the aplastic phase and promoting the reconstitution of the immune system.
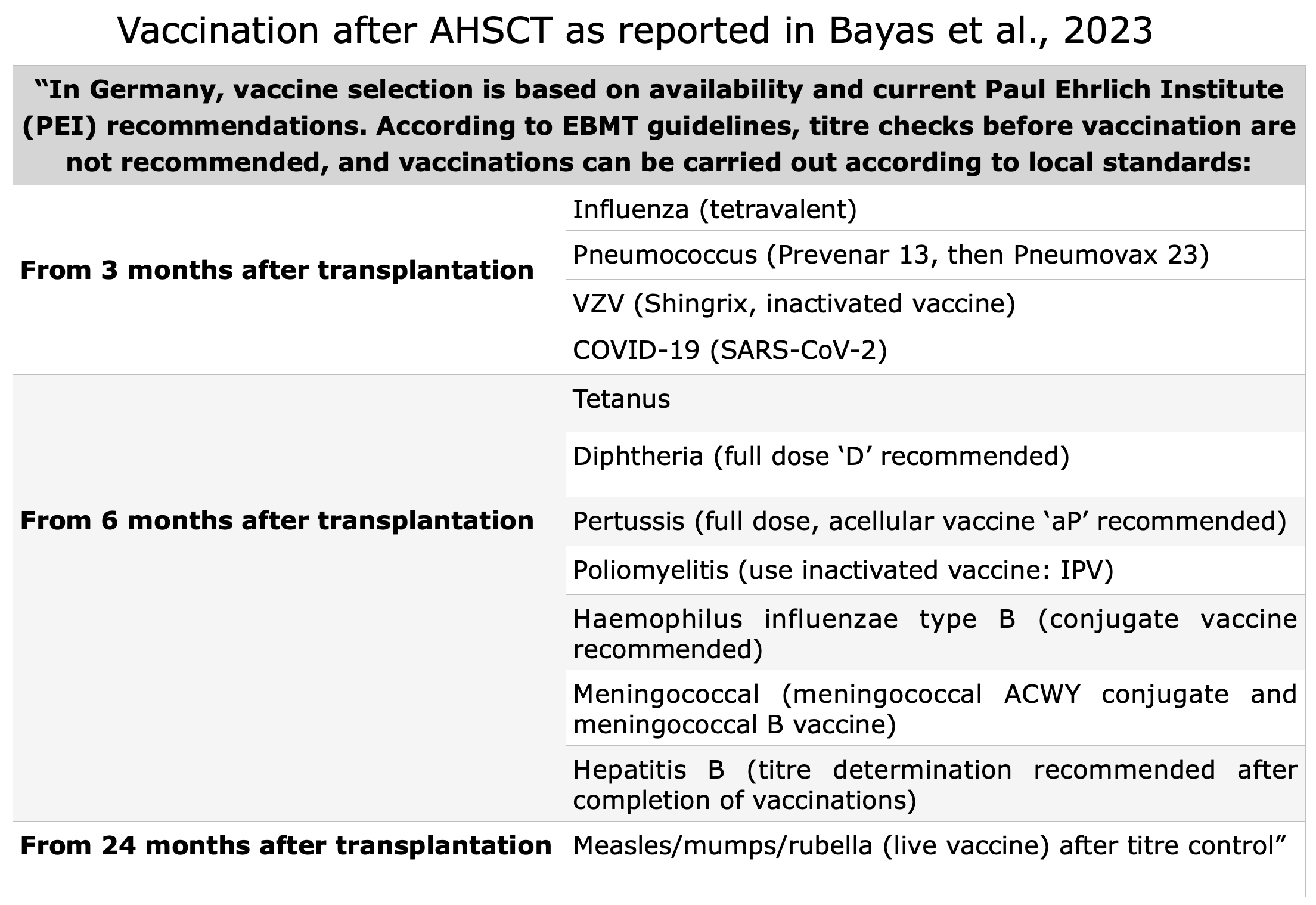
Long-Term Monitoring
Typically, three months after the transplant, the patient undergoes an initial MRI, followed by subsequent MRI every six months.
From the second year onward, the frequency is reduced to once per year, accompanied by both neurological and hematological evaluations. Specific blood tests are not required at this stage.
In the event of suspected relapse, a neurological evaluation is mandatory.
AHSCT Centers & Costs
Availability and reimbursement of AHSCT worldwide (list in progress)
Reimbursement criteria and availability vary between countries and may change over time.
Germany 2023: “In Germany, AHSCT for MS in general is not paid for by insurance companies and hence is only performed on an individual basis and expert opinion. Therefore, coverage needs to be negotiated from case-to-case and after rejection of compensation from insurance companies, patients need to pay on their own. Some try to reach out for a social court verdict.” (Bayas et al., 2023)
Italy: AHSCT is available and paid by the National Health Service (Mariottini et al., 2024)
Since December 2022, AHSCT is covered by basic health insurance but only for a highly specific patient group. Strict eligibility criteria apply: age 18-55, RRMS with disease duration <10 years, EDSS 3.0-6.5, and failed response to at least one highly effective DMT despite 6 months of treatment. Treatment is currently performed in two centers (Amsterdam UMC and St. Antonius Hospital Nieuwegein) following mandatory review by a National Indication Committee (Zorginstituut Nederland, 2022).
Sweden 2025: “In Sweden, AHSCT is approved by the Swedish Board of Health and Welfare for use in aggressive RRMS, whereas SPMS and PPMS are not endorsed.” (Mitrache Desaga et al., 2025)
USA: “The average total cost of care for inpatient aHSCT in the U.S. is $150,000. This usually includes the care required before and after the procedure. However, the cost of aHSCT varies widely. Prices are based on the patient and their treatment plan.Most insurance companies will probably not cover this procedure. Expect to pay for it out of pocket or enroll in a clinical trial where the costs are covered by the trial.” ” (National MS Society, 2025)
“HSCT mean total costs, based on our own hospital [Northwestern Memorial Hospital in Chicago], were $85,184 (range $70,635 to $120,260). Mean revenue collected was $95,268 (range $16,544 to $173,204). In comparison, according to the literature, 2019 DMT costs in the USA ranged from $80,000 to $100,000 per year per patient.” (Burt et al., 2020).
A detailed description of the procedure for obtaining a possible reimbursement is provided in Prof. R.K. Burt’s book (page 105: “Insurance approval” section).
Some Neurological and Hematological Centers that performed AHSCT in MS
The following is a list of centres worldwide that, to the best of our knowledge, perform AHSCT in MS (the list is not exhaustive and is continuously updated).
A center to be included in the list must satisfy the following criteria:
- Certification: by the European EBMT-JACIE, or equivalent international accreditation, such as the Joint Commission International (USA).
- Conditioning regimen: use intermediate-intensity regimens such as CY-ATG (non-myeloablative, also called lymphoablative) or BEAM-ATG (myeloablative), as reported in the consensus statement (Muraro et al., 2025).
The year of the latest scientific publication and the trial, if the center is involved, are reported in bold.
✅ Useful link: ongoing AHSCT trials here.
| Country |
Hospital |
|---|---|
| Australia | Department of Neurology, St. Vincent’s Hospital, Darlinghurst, 2023 |
| Canada | Department of Medicine (Neurology), The University of Ottawa and The Ottawa Hospital Research Institute, Ottawa, 2025 |
| Czech Republic | Department of Neurology, University Hospital Ostrava, 2023 |
| Denmark | Danish Multiple Sclerosis Center (DMSC), Copenhagen University Hospital – Rigshospitalet (Copenhagen), 2023 | RAM-MS Trial |
| Germany | University Medical Center Hamburg-Eppendorf, 2025 |
| India |
|
| Israel |
Hadassah University Hospital, Jerusalem, Ein-Kerem, 2020 |
| Italy |
|
| Lithuania |
Hematology, Oncology and Transfusion Medicine Center of Vilnius University Hospital Santaros Klinikos, Vilnius, 2020 |
| Netherlands |
|
| Norway |
Haukeland University Hospital, Bergen, 2025 | RAM-MS Trial |
| Russia |
Pirogov National Medical and Surgical Center, Moscow, 2022 |
| Spain |
Hospital Universitari La Fe Valencia and Hospital Clínic Universitari of Valencia, 2017 |
| Sweden |
|
| Switzerland |
Department of Hematology, University Hospital of Lausanne (CHUV), and University of Lausanne, Lausanne, Switzerland, 2023 |
| Turkey | Erciyes University Faculty of Medicine, Department of Internal Medicine Hematology, Kayseri, Turkiye, 2024 |
| United Arab Emirates |
Abu Dhabi Stem Cell Center, Abu Dhabi, United Arab Emirates, 2023 |
| UK |
|
| USA |
|
WARNING: SHAM STEM CELL CENTERS AND STEM CELL TOURISM
As suggested by the National MS Society USA (link here): “Some clinics in the U.S. and abroad do not have the necessary experience performing aHSCT on MS patients.
Some clinics overstate their experience online with testimonials to attract patients.
Make sure the clinics can back up their claims with peer-reviewed research. And consult your MS specialist. They can help ensure that the clinic follows strict health and safety standards.”
AHSCT: Patients' Stories
Below first-hand accounts of pwMS who have undergone AHSCT, as documented in the scientific literature:
Other Patients' Experiences
Below is a series of additional direct experiences shared by patients and collected from various MS Association websites:
Below are presented personal accounts through video narratives collected online:
For an immersive insight into patient’s journeys, we highly recommend reading “Everyday Miracles: Curing Multiple Sclerosis, Scleroderma, and Autoimmune Diseases by Hematopoietic Stem Cell Transplant” by Prof. R.K. Burt describes AHSCT patient’s journeys, following some testimonials of MS.

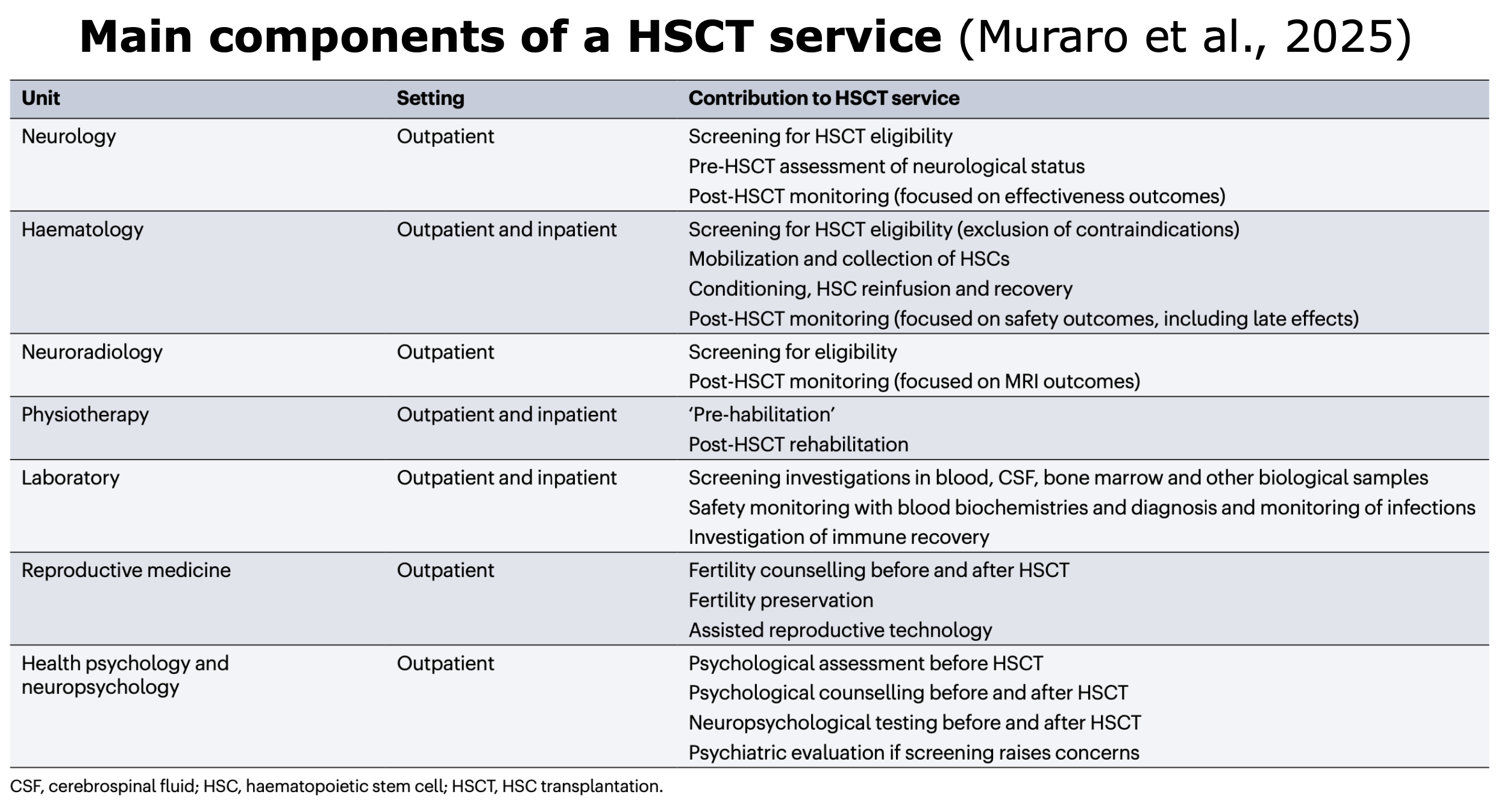

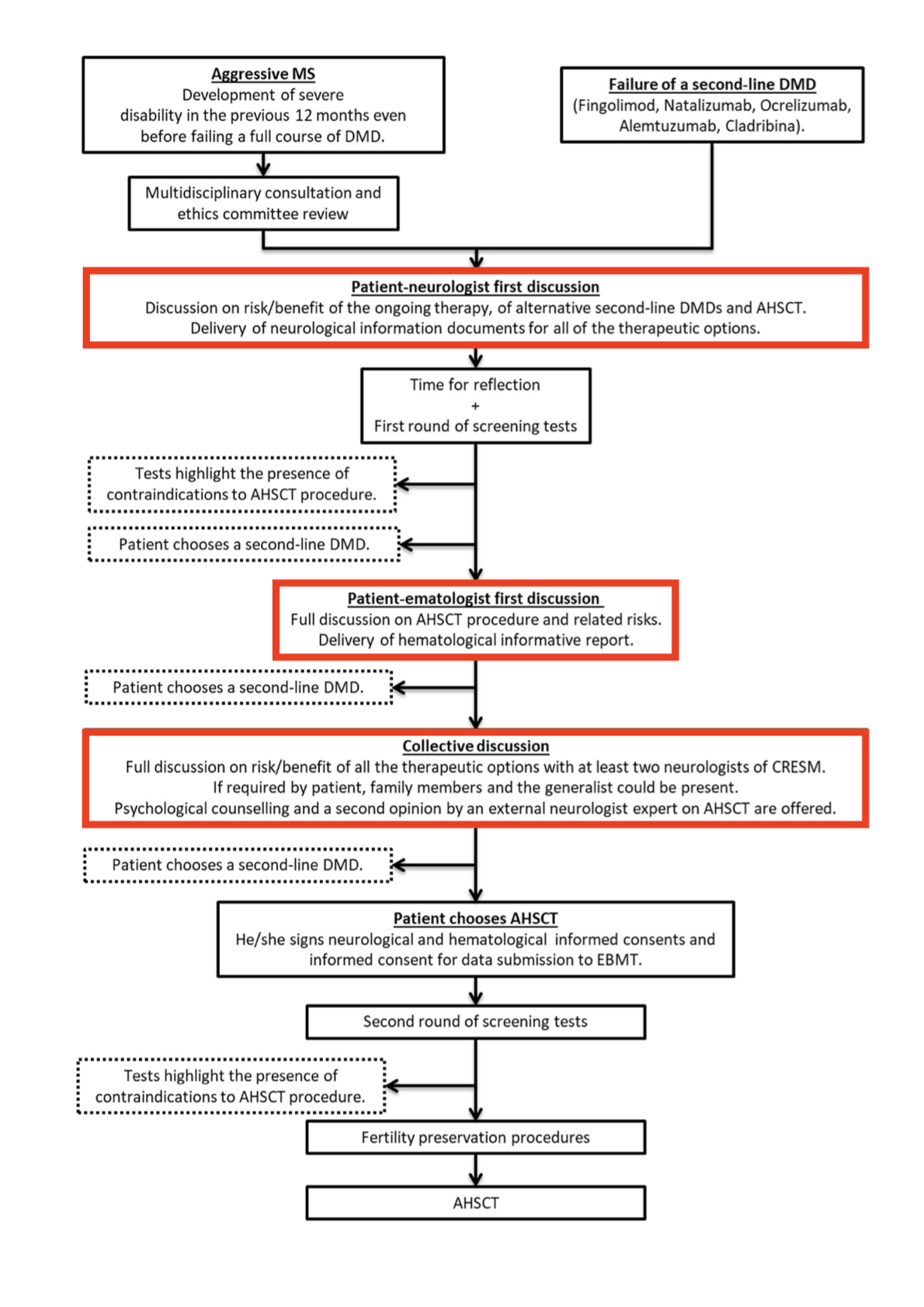 Figure from
Figure from